��Ŀ����
��֪��Ӧ��H2(g) �� 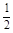 O2(g) �� H2O(g) ��H1
O2(g) �� H2O(g) ��H1
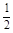 N2(g) �� O2(g) �� NO2 (g) ��H2
N2(g) �� O2(g) �� NO2 (g) ��H2
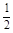 N2(g) ��
N2(g) �� 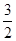 H2(g) �� NH3 (g) ��H3
H2(g) �� NH3 (g) ��H3
��Ӧ2NH3 (g) �� O2(g) ��2NO2 (g) ��3H2O(g) �ġ�H��
O2(g) ��2NO2 (g) ��3H2O(g) �ġ�H��
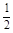 O2(g) �� H2O(g) ��H1
O2(g) �� H2O(g) ��H1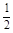 N2(g) �� O2(g) �� NO2 (g) ��H2
N2(g) �� O2(g) �� NO2 (g) ��H2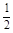 N2(g) ��
N2(g) �� 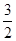 H2(g) �� NH3 (g) ��H3
H2(g) �� NH3 (g) ��H3��Ӧ2NH3 (g) ��
 O2(g) ��2NO2 (g) ��3H2O(g) �ġ�H��
O2(g) ��2NO2 (g) ��3H2O(g) �ġ�H��| A��2��H1��2��H2��2��H3 | B����H1����H2����H3 |
| C��3��H1��2��H2��2��H3 | D��3��H1��2��H2��2��H3 |
D
�������������ʽ�١�3+�ڡ�2���ۡ�2�õ���Ӧ2NH3 (g) ��
 O2(g) ��2NO2 (g) ��3H2O(g) �����Ը÷�Ӧ�ġ�H��3��H1��2��H2��2��H3��
O2(g) ��2NO2 (g) ��3H2O(g) �����Ը÷�Ӧ�ġ�H��3��H1��2��H2��2��H3��
��ϰ��ϵ�д�
�����Ŀ

 CH3OCH3(g) + CO2(g)����H��-247kJ/mol
CH3OCH3(g) + CO2(g)����H��-247kJ/mol
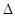 H1=" +1175.7" kJ·mol-1
H1=" +1175.7" kJ·mol-1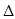 H3=" +482.2" kJ·mol-1
H3=" +482.2" kJ·mol-1
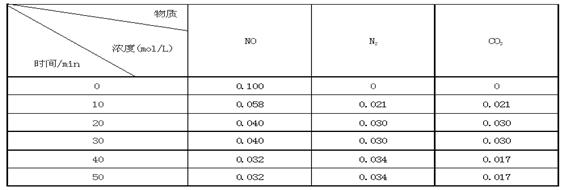
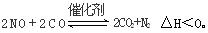
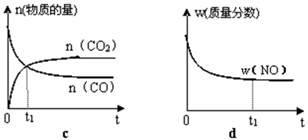
 C(s)=
C(s)= 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0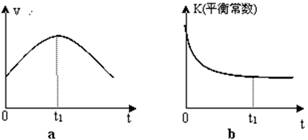
 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol