题目内容
已知:
(1)Fe2O3(s) + C(s)=
C(s)= CO2(g)+2Fe(s) ΔH=+234.1 kJ/mol
CO2(g)+2Fe(s) ΔH=+234.1 kJ/mol
(2)C(s)+O2(g)=CO2(g) ΔH=-393.5 kJ/mol
则2Fe(s)+ O2(g)=Fe2O3(s) 的ΔH是( )
O2(g)=Fe2O3(s) 的ΔH是( )
(1)Fe2O3(s) +
 C(s)=
C(s)= CO2(g)+2Fe(s) ΔH=+234.1 kJ/mol
CO2(g)+2Fe(s) ΔH=+234.1 kJ/mol(2)C(s)+O2(g)=CO2(g) ΔH=-393.5 kJ/mol
则2Fe(s)+
 O2(g)=Fe2O3(s) 的ΔH是( )
O2(g)=Fe2O3(s) 的ΔH是( )| A.-824.4 kJ/mol | B.-627.6 kJ/mol |
| C.-744.7 kJ/mol | D.-169.4 kJ/mol |
A
试题分析:2Fe(s)+
 O2(g)=Fe2O3(s)可由(2)×2/3-(1)得到,所以其ΔH=-393.5×2/3-234,答案为A.
O2(g)=Fe2O3(s)可由(2)×2/3-(1)得到,所以其ΔH=-393.5×2/3-234,答案为A.
练习册系列答案
 世纪百通主体课堂小学课时同步达标系列答案
世纪百通主体课堂小学课时同步达标系列答案
相关题目
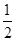 H2(g)+
H2(g)+  2NO2(g) ΔH 的体系中,n(NO)随时间的变化如表:
2NO2(g) ΔH 的体系中,n(NO)随时间的变化如表: O2(g) = H2O(g) △H1
O2(g) = H2O(g) △H1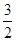 H2(g) = NH3 (g) △H3
H2(g) = NH3 (g) △H3 O2(g) =2NO2 (g) +3H2O(g) 的△H=
O2(g) =2NO2 (g) +3H2O(g) 的△H=
 H= ?241.8kJ/mol
H= ?241.8kJ/mol  2SO3(g);
2SO3(g); 2NO(g)
2NO(g)  CH3OH(g);CO的平衡转化率(α)与温度、压强的关系如图所示。
CH3OH(g);CO的平衡转化率(α)与温度、压强的关系如图所示。
 键分别需要的能量是436kJ、391KJ、946kJ,则N2与H2反应生成1mol NH3(g)的热化学方程式是___________________.
键分别需要的能量是436kJ、391KJ、946kJ,则N2与H2反应生成1mol NH3(g)的热化学方程式是___________________.
 CO2(g)+H2(g) △H=-41.2kJ·mol-1
CO2(g)+H2(g) △H=-41.2kJ·mol-1 CH3CH2OH(g)+3H2O(l) △H= 。
CH3CH2OH(g)+3H2O(l) △H= 。

 CO(g)+3H2(g),试回答下列问题。
CO(g)+3H2(g),试回答下列问题。