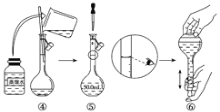
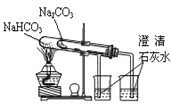
��Ŀ����
����Ŀ����ͼ����4��̼ԭ�ӽ�ϳɵ�6���л����ԭ��û�л�������
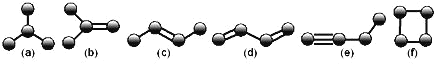
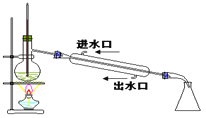
��1��д���л���(a)��ϵͳ������������_____��
��2���л���(e)��һ��ͬ����ͬ���칹�壬��д����ṹ��ʽ_____��
��3�������л�������(c)��Ϊͬ���칹�����_____������ţ���
��4��дһ����(b)��Ϊͬϵ��������̼ԭ�������ٵ��л���Ľṹ��ʽ_____��
��5�������л����в�������ˮ��Ӧʹ����ɫ����_____������ţ���
��6��д���л���(d)��Ӧ���ɸ߷��ӻ�����Ļ�ѧ����ʽ_____��
���𰸡�2-������ CH3-C��C-CH3 bf CH2=CH2 af nCH2=CH-CH=CH2![]()
��������
��a��Ϊ2-�����飬��b��Ϊ2-����ϩ����c��Ϊ2-��ϩ����d��Ϊ1,3-����ϩ����e��Ϊ1-��Ȳ����f��Ϊ�����飬�ݴ���������С�⼴�ɡ�
��1���л��a����һ����֧���ı��飬���Ϊ2-�����飻
��2��eΪ1-��Ȳ��ͬ����ͬ���칹��ҲΪȲ����2-��Ȳ����ṹ��ʽΪ![]() ��
��
��3�����ԴӲ����ͶȵĽǶ������ǣ���c���н���1�������Ͷȣ�����b����f��Ҳ��ֻ��1�������Ͷȣ�
��4����b��Ϊ��ϩ������ĵ�ϩ��Ϊ��ϩ����ṹ��ʽΪ![]() ��
��
��5����a���ͣ�f�������ͼ�����˲�������ˮ������Ӧ��
��6��d���Է����Ӿ۷�Ӧ�õ��߷��ӻ�����䷴Ӧ����ʽΪnCH2=CH-CH=CH2![]() ��
��

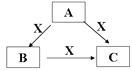
 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�����Ŀ�������ֶ�����Ԫ�أ����ǵĽṹ�����ʵ���Ϣ���±�������
Ԫ�� | �ṹ�����ʵ���Ϣ |
A | �Ƕ������У���ϡ�������⣩ԭ�Ӱ뾶����Ԫ�أ���Ԫ�ص�ij�ֺϽ���ԭ�ӷ�Ӧ�ѵĵ��ȼ� |
B |
|
C | Ԫ�ص���̬�⻯�K������ˮ������������� |
D | �Ǻ�ˮ�г��⡢��Ԫ���⺬������Ԫ�أ��䵥�ʻ���Ҳ������ˮ���������г��õ�����ɱ���� |
����ݱ�����Ϣ��д��
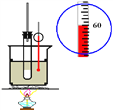
��1��![]() ԭ�ӵĺ�������Ų�ʽ��___________________��
ԭ�ӵĺ�������Ų�ʽ��___________________��
��2��![]() Ԫ����Ԫ�����ڱ��е�λ��________�����Ӱ뾶��
Ԫ����Ԫ�����ڱ��е�λ��________�����Ӱ뾶��![]() ____
____![]() ����������������������
����������������������
��3��![]() ԭ�ӵĵ����Ų�ͼ��______��������ߵĵ���Ϊ______����ϵĵ��ӣ�������______�Ρ�
ԭ�ӵĵ����Ų�ͼ��______��������ߵĵ���Ϊ______����ϵĵ��ӣ�������______�Ρ�
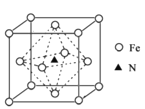
��4��![]() �Ľṹʾ��ͼ��________________��
�Ľṹʾ��ͼ��________________��
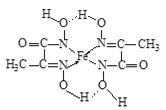
��5��![]() ������������Ӧ��ˮ������
������������Ӧ��ˮ������![]() ������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪ________����
������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪ________����![]() ����������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪ________________��
����������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪ________________��
��6����һʵ��˵��![]() Ԫ�صķǽ����Ա�
Ԫ�صķǽ����Ա�![]() Ԫ�ص�ǿ_______________
Ԫ�ص�ǿ_______________