��Ŀ����
4�� A��B��C��D��E��ԭ���������ε����Ķ�����Ԫ�أ�A+���ӣ�BD��C2�ǵȵ����壬��һ�����ܣ�C��D��B��D��Eͬ���壬ED2���γ��������ҪΣ�����壬FԪ��λ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ2��
A��B��C��D��E��ԭ���������ε����Ķ�����Ԫ�أ�A+���ӣ�BD��C2�ǵȵ����壬��һ�����ܣ�C��D��B��D��Eͬ���壬ED2���γ��������ҪΣ�����壬FԪ��λ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ2����ش��������⣺
��1��C2���ӵĵ���ʽ��
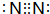 ��E����Ԫ�����ڱ���ds��
��E����Ԫ�����ڱ���ds����2��A��B��C��D�γɵ���״��ԭ�ӷ�����ֻ��Aԭ�������δ�ﵽ8e-�ṹ���÷��ӵĽṹʽ������H-O-C��N
��3��E��F���γɻ����ᄃ��ľ�����ͼ��ʾ���þ���Ļ�ѧʽ��ZnS
��4��C2A4���ӵ�����ԭ���ӻ���ʽ��sp3����ˮ��Һ�Լ��Ե�ԭ����N2N4+H2O?N2N5++OH-�������ӷ���ʽ��ʾ��
��5����֪A-A����Ϊ436kJ/mol��C-A����Ϊ391kJ/mol�����ݷ�Ӧ�Ļ�ѧ����ʽ��C2��g��+3A2��g��?2CA3��g����H=-92.4kJ/mol����C��C�ļ��ܣ��������945.6kJ/mol��
���� A��B��C��D��E��ԭ���������ε����Ķ�����Ԫ�أ�A+���ӣ���AΪHԪ�أ�D��Eͬ���壬ED2���γ��������ҪΣ�����壬��EΪSԪ�ء�DΪOԪ�أ�BD��C2�ǵȵ����壬��B��C��DΪ���ڵ�����Ԫ�أ���һ�����ܣ�C��D��B����C���ڢ�A�壬��BΪ̼Ԫ�ء�CΪNԪ�أ�FԪ��λ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ2�����������Ϊ2+8+18+2=30����FΪZn���ݴ˽��
��� �⣺A��B��C��D��E��ԭ���������ε����Ķ�����Ԫ�أ�A+���ӣ���AΪHԪ�أ�D��Eͬ���壬ED2���γ��������ҪΣ�����壬��EΪSԪ�ء�DΪOԪ�أ�BD��C2�ǵȵ����壬��B��C��DΪ���ڵ�����Ԫ�أ���һ�����ܣ�C��D��B����C���ڢ�A�壬��BΪ̼Ԫ�ء�CΪNԪ�أ�FԪ��λ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ2�����������Ϊ2+8+18+2=30����FΪZn��
��1��N2������Nԭ���γ�3�Թ��õ��Ӷԣ������ʽ��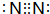 ��EΪZnԪ�أ����ڵ������ڢ�B�壬����Ԫ�����ڱ���ds����
��EΪZnԪ�أ����ڵ������ڢ�B�壬����Ԫ�����ڱ���ds����
�ʴ�Ϊ��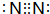 ��ds��
��ds��
��2��H��C��N��O�γɵ���״��ԭ�ӷ���ΪHCNO��ֻ��Hԭ�������δ�ﵽ8e-�ṹ��Oԭ���γ�2�����ۼ���Cԭ���γ�4�����ۼ���Nԭ���γ�3�����ۼ�����ṹʽΪH-O-C��N��
�ʴ�Ϊ��H-O-C��N��
��3��������Znԭ����ĿΪ4��Sԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4���ʸþ���Ļ�ѧʽ��ZnS��
�ʴ�Ϊ��ZnS��
��4��N2N4������Nԭ���γ�3���Ҽ�������1�Թµ��Ӷԣ�Nԭ�Ӳ�ȡsp3�ӻ�����ˮ��Һ�Լ��Ե�ԭ���ǣ�N2N4+H2O?N2N5++OH-��
�ʴ�Ϊ��sp3��N2N4+H2O?N2N5++OH-��
��5����֪H-H����Ϊ436kJ/mol��N-H����Ϊ391kJ/mol���Ȼ�ѧ����ʽ��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol����EN��N+3��436kJ/mol-2��3��391kJ/mol=-92.4kJ/mol�����EN��N=945.6kJ/mol��
�ʴ�Ϊ��945.6kJ/mol��
���� �����Ƕ����ʽṹ�Ŀ��飬�ƶ�Ԫ���ǽ���ؼ����漰����ʽ��Ԫ�����ڱ������ӽṹ���������㡢��Ӧ���йؼ���ȣ��Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
��1��������[Cu��En��2]2+���������ӻ�̬��Χ�����Ų�ʽΪ3d9���Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊsp3��������[Cu��En��2]2+�е���λ��Ϊ4��
��2���Ҷ��������װ�[N��CH3��3]�����ڰ��࣬���Ҷ��������װ��ķе�ߵĶ࣬ԭ�����Ҷ�������֮������γ���������װ�����֮�䲻���γ������
��3���Ƚϱ����д�С���á����ڡ���С�ڡ������ڡ���գ�
| ��һ������ | ���Ӱ뾶 | �۵� | ���� |
| P��S | H-��Li+ | KCl��Si | HClO3��HClO4 |
A��ͼ��aλ����Clԭ��Ϊsp�ӻ������
B��M�Ļ�ѧʽȷ����KCuCl3����N�Ļ�ѧʽΪK2CuCl3��
C��������[CuCl4]2-��������[AlCl4]-�ռ�ṹ���ƣ�
����ͭ�����백ˮ����������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ��Ӧ����Ӧ�����ӷ�Ӧ����ʽΪCu+H2O2 +4NH3�TCu��NH3��42++2OH-��
��5�����������ʵľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������ֱ���ͼ3��ʾ������֪�������������������ľ����ѻ��Ŀռ������ʷֱ�Ϊ68%��74%������ʽ�������־�����ܶ�֮��[�ѣ�a�����ѣ�b��]Ϊ3$\sqrt{3}$��4$\sqrt{2}$������ɼĹ�ϵʽ�������������ֻ��д������÷֣���
| A�� | ������һ�������� | |
| B�� | 1�������������3��̼ԭ�Ӻ�3��ˮ���ӹ��� | |
| C�� | ��������������������ԭ��������ͬ | |
| D�� | �����Dz���Ϊ�����ṩ���� |

| A�� | ��ͼ��֪�������¶ȴ����Ƶ�ˮ��̶����� | |
| B�� | ��ͼ�ҿ�֪��a��Kw����ֵ��b��Kw����ֵ�� | |
| C�� | ��ͼ����֪����ӦA��g��+B��g��=2C��g�������ȷ�Ӧ | |
| D�� | ��ͼ����֪����ӦC�����ʯ��s��=C��ʯī��s�����ʱ��H=��H1-��H2 |
| A�� | 0.1 mol����ϩ�к���̼̼˫������ĿΪ0.4NA | |
| B�� | ��1molCl2ͨ�뵽ˮ�У���N��HClO��+N��Cl-��+N��ClO-��=2[NA-N��Cl2��] | |
| C�� | һ�������£�0.1 mol SO2������������Ӧ����SO3��ת�Ƶ�����Ϊ0.2NA | |
| D�� | ��⾫��ͭ������·��ͨ���ĵ�����ĿΪ0.2NAʱ��������������6.4g |
| �� �� | �� �� | �� �� | |
| A | ��ij��Һ�еμ�BaCl2��Һ��ϡHNO3 | ������ɫ���� | ��Һ��һ������SO42- |
| B | ���ۺ�ϡ�����Ϲ��Ⱥ��ټ���������������ͭ����Һ | ������ɫ���� | ����ˮ������������� |
| C | ��ʢ��0.5mol•L-1Fe��NO3��2��Һ���Թ��м���0.5mol•L-3H2SO4��Һ | ���Թܿڴ��ֺ���ɫ���� | ��Һ��NO3-��Fe2+��ԭΪNO2 |
| D | ��ij��ɫ��Һ�еμ���ˮ��CCl4�������� | �²���Һ����ɫ | ԭ��Һ����I- |
| A�� | A | B�� | B | C�� | C | D�� | D |
| R | T | |
| X | Y | Z |
| A�� | Rλ��Ԫ�����ڱ��еڶ����ڵ�VIA�� | |
| B�� | ����Ԫ����ԭ������������X | |
| C�� | ��̬�⻯���ȶ��ԣ�T��Y | |
| D�� | ZԪ�ص�����������Ӧ��ˮ����Ļ�ѧʽΪH2ZO4 |
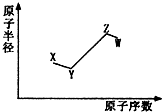 �����ڷǽ�������Ԫ��X��Y��Z��W�����ǵ�ԭ�Ӱ뾶��ԭ�������Ĺ�ϵ��ͼ��ʾ������ZԪ��ԭ��������Y��2����ԭ��������������XԪ��ԭ�Ӻ����������ȣ�����˵��һ����ȷ���ǣ�������
�����ڷǽ�������Ԫ��X��Y��Z��W�����ǵ�ԭ�Ӱ뾶��ԭ�������Ĺ�ϵ��ͼ��ʾ������ZԪ��ԭ��������Y��2����ԭ��������������XԪ��ԭ�Ӻ����������ȣ�����˵��һ����ȷ���ǣ�������| A�� | X��Z��Y��W����ͬһ���� | |
| B�� | 4��Ԫ���γɵĸ��ֵ����У�Z���ʵķе���� | |
| C�� | W������X��Y��Z������⻯�ﶼ���Է�����Ӧ | |
| D�� | Ԫ������������Ӧ��ˮ�������ԣ�W��Z��X |
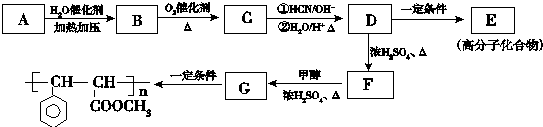
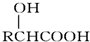
 ��
�� ��
��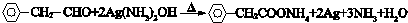 ��
�� ��
��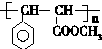 ����������������Һ��Ӧ�Ļ�ѧ����ʽ
����������������Һ��Ӧ�Ļ�ѧ����ʽ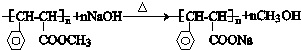 ��
��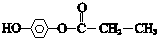 ��
��