��Ŀ����
(14��)J��L��M�� R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�ء�
R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�ء�
��1��M�����ӽṹʾ��ͼΪ_____;Ԫ��T���ڱ���λ�ڵ�___�塣
��2��J������ɵĻ����������4��ԭ�ӣ���ṹʽΪ____ __ ��
��
(3)M��T�γɵĻ������� ��ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ___ __��
��ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ___ __��
��4��L�������̬�⻯���ˮ��Һ�Լ��ԡ�
�������ӹ�ҵ�У���ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ_ __ ___��
���ɼ���������KOH ��Һ����ԭ��أ����������L�ĵ��ʡ����为����ӦʽΪ__________________________ ____��
��5����J��R�γɵ�Һ̬������JR2 0��2mol��O2����ȫȼ�գ�����������̬�����298Kʱ�ų�����215kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ____ ____��
 R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�ء�
R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�ء�
��1��M�����ӽṹʾ��ͼΪ_____;Ԫ��T���ڱ���λ�ڵ�___�塣
��2��J������ɵĻ����������4��ԭ�ӣ���ṹʽΪ____ __
 ��
��(3)M��T�γɵĻ�������
 ��ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ___ __��
��ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ___ __����4��L�������̬�⻯���ˮ��Һ�Լ��ԡ�
�������ӹ�ҵ�У���ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ_ __ ___��
���ɼ���������KOH ��Һ����ԭ��أ����������L�ĵ��ʡ����为����ӦʽΪ__________________________ ____��
��5����J��R�γɵ�Һ̬������JR2 0��2mol��O2����ȫȼ�գ�����������̬�����298Kʱ�ų�����215kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ____ ____��
��14�֣��� 1�� ��2�֣���A��2�֣�
1�� ��2�֣���A��2�֣�
��2�� ��2��
��2��
��3��AlCl3 +3H2O =Al(OH)3 +3HCl����2�֣�
��4����NH3��H2O + 3H2O2 =N2 +8H2O��2�֣�
��2NH3 һ 6e��+ 6OH�� ="=" N2 + 6H2O��2�֣�
( 5 ) CS2��l��+3O2��g��=CO2��g��+2SO2��g����H=-1075KJ/mol��2�֣�
 1�� ��2�֣���A��2�֣�
1�� ��2�֣���A��2�֣���2��
 ��2��
��2����3��AlCl3 +3H2O =Al(OH)3 +3HCl����2�֣�
��4����NH3��H2O + 3H2O2 =N2 +8H2O��2�֣�
��2NH3 һ 6e��+ 6OH�� ="=" N2 + 6H2O��2�֣�
( 5 ) CS2��l��+3O2��g��=CO2��g��+2SO2��g����H=-1075KJ/mol��2�֣�
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
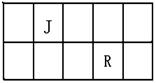
 E�������ӽṹʾ��ͼ�� (1��)
E�������ӽṹʾ��ͼ�� (1��) ������ͨ��1L
������ͨ��1L  1mol��L-1
1mol��L-1
 Ԫ��ԭ�ӵ��������������������������=3/4����C��Eͬ���塣
Ԫ��ԭ�ӵ��������������������������=3/4����C��Eͬ���塣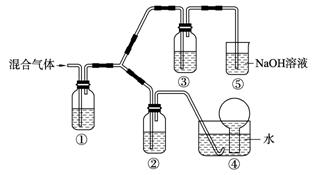
 ________________________________________��
________________________________________�� �ʣ��ҷ�Ӧ����ˮ��Һ�н��С�
�ʣ��ҷ�Ӧ����ˮ��Һ�н��С� �����ֳ���Ԫ��W��X��Y��Z�������γ�һ�ֻ�������������W��Y���������ǵ����������Ҫ���ʣ�X��������ȿ��Ժ�ǿ�ᡢ����Ժ�ǿ�Ӧ��Z�����ש��ɫ�ͺ�ɫ����
�����ֳ���Ԫ��W��X��Y��Z�������γ�һ�ֻ�������������W��Y���������ǵ����������Ҫ���ʣ�X��������ȿ��Ժ�ǿ�ᡢ����Ժ�ǿ�Ӧ��Z�����ש��ɫ�ͺ�ɫ���� �������
������� ���õ���ʽ��ʾ����̬��
���õ���ʽ��ʾ����̬�� ���� ��
���� �� ��
��