��Ŀ����
��12�֣���֪Ԫ�ص�ij�����ʡ�X����ԭ�Ӱ뾶�������ԡ��ǽ����Ե�һ����Ҳ��Ԫ�ص�һ�ֻ������ʡ��������13��Ԫ�ص�X����ֵ��
�Խ��Ԫ��������֪ʶ����������⣺
��1��������ɸ������ǣ����γɻ�ѧ������ԭ����ӦԪ�ص�X��ֵ����1.7ʱ�����γɵ�һ��Ϊ���Ӽ�����С��1.7ʱ��һ��Ϊ���ۼ������ƶ�BeCl2�еĻ�ѧ�������� ��
��2�������ϱ����������ݣ���������Ԫ�ص�X����ֵ��С��Ԫ�صĽ�����֮��Ĺ�ϵ �������ڶ�����Ԫ��(������������)��X����ֵ��С��ԭ�Ӱ뾶֮��Ĺ�ϵ ��
��3��ij����������к���S��N��������Ϊ�ù��õ��Ӷ�ƫ���� ԭ��(��Ԫ�ط���)��
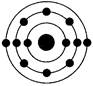
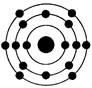
��4��д��Oԭ�ӵĵ����Ų�ͼ
��5��ClԪ�ص��������Ϊ ��������������ˮ����Ļ�ѧʽΪ ��
��6����Ҫ����ij���������Ƿ���SԪ�أ����� �ķ�������Ҫȷ��C2H6O�Ľṹ������ �ķ���������ʽΪC2H6O�����ֲ�ͬ�Ľṹ��ʽ�ֱ�Ϊ �� �������ֽṹ��Ϊ ��
| Ԫ�� | Al | B | Be | C | Cl | F | Li |
| X����ֵ | 1.5 | 2.0 | 1.5 | 2.5 | 2.8 | 4.0 | 1.0 |
| Ԫ�� | Mg | Na | O | P | S | Si | |
| X����ֵ | 1.2 | 0.9 | 3.5 | 2.1 | 2.5 | 1.7 | |
��1��������ɸ������ǣ����γɻ�ѧ������ԭ����ӦԪ�ص�X��ֵ����1.7ʱ�����γɵ�һ��Ϊ���Ӽ�����С��1.7ʱ��һ��Ϊ���ۼ������ƶ�BeCl2�еĻ�ѧ�������� ��
��2�������ϱ����������ݣ���������Ԫ�ص�X����ֵ��С��Ԫ�صĽ�����֮��Ĺ�ϵ �������ڶ�����Ԫ��(������������)��X����ֵ��С��ԭ�Ӱ뾶֮��Ĺ�ϵ ��
��3��ij����������к���S��N��������Ϊ�ù��õ��Ӷ�ƫ���� ԭ��(��Ԫ�ط���)��
��4��д��Oԭ�ӵĵ����Ų�ͼ
��5��ClԪ�ص��������Ϊ ��������������ˮ����Ļ�ѧʽΪ ��
��6����Ҫ����ij���������Ƿ���SԪ�أ����� �ķ�������Ҫȷ��C2H6O�Ľṹ������ �ķ���������ʽΪC2H6O�����ֲ�ͬ�Ľṹ��ʽ�ֱ�Ϊ �� �������ֽṹ��Ϊ ��
.(1)���ۼ� ��2��XԽ������Խ����XԽ�뾶ԽС ��3��N
��4��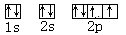 ��5��+7��HClO4 ��6��ԭ�ӹ��ף����ף�
��5��+7��HClO4 ��6��ԭ�ӹ��ף����ף�
CH3CH2OH��CH3-O-CH3��ͬ���칹��
��4��
 ��5��+7��HClO4 ��6��ԭ�ӹ��ף����ף�
��5��+7��HClO4 ��6��ԭ�ӹ��ף����ף�CH3CH2OH��CH3-O-CH3��ͬ���칹��
��1�����ݱ������ݿ�֪��XӦ����Ԫ�صĵ縺�ԡ�������Ԫ�غ�BeԪ�صĵ縺���γ�1.3�������Ȼ����л�ѧ���ǹ��ۼ���
��2������Ԫ�صĵ縺�Կ�֪��FԪ�صĵ縺�������Ԫ�صĵ縺����С�����Ե縺��Խ������Խ�����ǽ�����Խǿ�����ڵڶ�������FԪ�ص�ԭ�Ӱ뾶��С����Li��ԭ�Ӱ뾶���ͬ������������ԭ�Ӱ뾶����С�ģ����Ե縺��Խ��ԭ�Ӱ뾶ԽС��
��3���ǽ�����Խǿ���縺��Խ�����Ե�Ԫ�صĵ縺�Դ���̼Ԫ�صģ�������2.5�����Ե�Ԫ�صĵ縺��Ҳ����SԪ�صģ�N��S���й��õ��Ӷ�ƫ��Ԫ�ء�
��4�����ݹ���ԭ����֪����Ԫ�صĵ����Ų�ͼ�� ��
��
��5����Ԫ���ǵ���AԪ�أ���������ǣ�7�ۣ�������������ˮ����Ļ�ѧʽ��HClO4��
��2������Ԫ�صĵ縺�Կ�֪��FԪ�صĵ縺�������Ԫ�صĵ縺����С�����Ե縺��Խ������Խ�����ǽ�����Խǿ�����ڵڶ�������FԪ�ص�ԭ�Ӱ뾶��С����Li��ԭ�Ӱ뾶���ͬ������������ԭ�Ӱ뾶����С�ģ����Ե縺��Խ��ԭ�Ӱ뾶ԽС��
��3���ǽ�����Խǿ���縺��Խ�����Ե�Ԫ�صĵ縺�Դ���̼Ԫ�صģ�������2.5�����Ե�Ԫ�صĵ縺��Ҳ����SԪ�صģ�N��S���й��õ��Ӷ�ƫ��Ԫ�ء�
��4�����ݹ���ԭ����֪����Ԫ�صĵ����Ų�ͼ��
 ��
����5����Ԫ���ǵ���AԪ�أ���������ǣ�7�ۣ�������������ˮ����Ļ�ѧʽ��HClO4��

��ϰ��ϵ�д�
 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
�����Ŀ

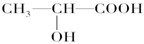
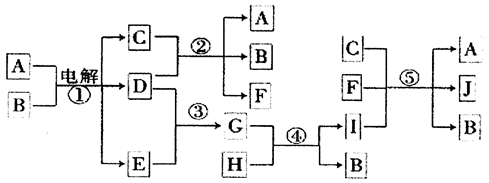
 R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�ء�
R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�ء�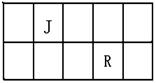
 ��
�� ��ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ___ __��
��ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ___ __��

 __________________��
__________________��