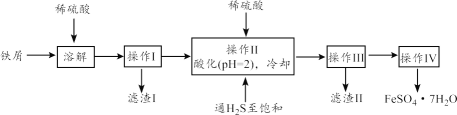
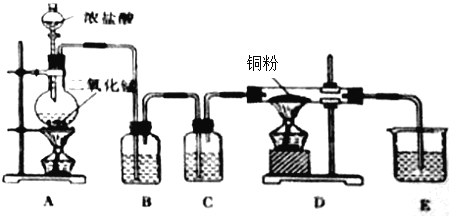
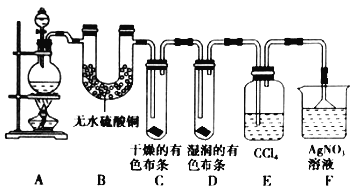
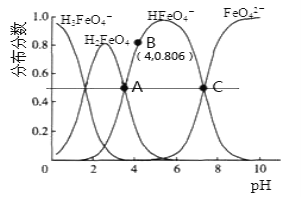
��Ŀ����
����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�~���ڱ��е�λ�ã��ش��������⣺

(1)�ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ����_______��
(2)�ڵ����������ķ���ʽΪ____��
(3)�١��ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д������Ҫ���һ�ֻ�����ĵ���ʽ_____��
(4)W�ǵ����������ͬ�����Ԫ�ء��ݴ��Ʋ�W�����ܾ��е�������___
A.��������ϼ�Ϊ+6 B.��̬�⻯���H2S�ȶ�
C.����������Ӧˮ��������Ա������� D.�����ڳ����¿�����������
(5)��֪XΪ�ڢ�A��Ԫ��(��һ����������)����ԭ������Ϊa��Y��Xλ��ͬһ���ڣ���Ϊ�ڢ�A��Ԫ�أ���Y��ԭ������b��a���п��ܵĹ�ϵʽΪ____��
���𰸡��������ڵڢ�A�� CO2 NaOH��![]() ��Na2O2��
��Na2O2��![]() BD b=a+1��b=a+11
BD b=a+1��b=a+11
��������
��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
(1)�ؿ��к������ڵڶ�λ��Ԫ��ΪSi��
(2)�ڱ�ʾCԪ�أ�����Ԫ������ϼ۵���ԭ����������������ԭ������������
(3)��H��O��Na�е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ�������NaOH��Na2O2�ȣ�
(4)W�ǵ����������ͬ����Ԫ�أ�����OԪ�أ���WΪSeԪ�أ�����Ԫ�������ɷ����жϣ�
(5)����Ԫ�����ڱ���λ����ԭ��������ϵ�������
��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
(1)�ؿ��к������ڵڶ�λ��Ԫ��ΪSi��Siԭ�Ӻ�������Ų�Ϊ2��8��4������Si����Ԫ�����ڱ��е������ڵڢ�A�壻
(2)�ڱ�ʾCԪ�أ�Cԭ���������4�����ӣ����������������ķ���ʽΪCO2��
(3)��H��O��Na�е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ�������NaOH��Na2O2�ȣ�����NaOH�ĵ���ʽΪ��![]() ��Na2O2�ĵ���ʽΪ��
��Na2O2�ĵ���ʽΪ��![]() ��
��
(4)W�ǵ����������ͬ����Ԫ�أ�����OԪ�أ���WΪSeԪ�ء�
A. Seԭ���������6�����ӣ���������������ϼ�Ϊ+6��A��ȷ��
B. Ԫ�صķǽ�����Խǿ������Ӧ�ļ��⻯����ȶ��Ծ�Խǿ������Ԫ�صķǽ����ԣ�S>Se��������̬�⻯���ȶ��ԣ�H2S>H2Se��B����
C. ͬһ�����Ԫ��ԭ������Խ��Ԫ�صķǽ�����Խǿ��������������Ӧˮ���������Խǿ�����ڷǽ�����S>Se������H2SeO4<H2SO4��C��ȷ��
D. Ԫ�صķǽ�����Խǿ���䵥��Խ�������������ϣ�����Ԫ�صķǽ����ԣ�S>Se��S��H2��Ӧ���ڼ��������½��У���Se������H2��ӦҪ���¶Ȼ���ߣ��ڳ����²�������H2���ϣ�D����
�ʺ���ѡ����BD��
(5)Xԭ������Ϊa��Yԭ������Ϊb����Xλ�ڵڶ����ڵڢ�A��Ԫ�أ���Xλ�ڵ������ڵ�IIA�壬����ͬһ���ڵ�IIIA��Ԫ��Yԭ������Ϊb=a+1����Xλ�ڵ������ڵڢ�A��Ԫ�أ����ڵ�IIA�塢��IIIA��֮��������7�������1����VIII��Ԫ�أ���10���У�������ͬһ���ڵ�IIIA��Ԫ��Yԭ������Ϊb=a+10+1=a+11��

 ��У����ϵ�д�
��У����ϵ�д�