��Ŀ����
����Ŀ����һ����![]() ��Ũ������ȡ�������������������������ͭ�۷�Ӧ��ȡ��������ˮ
��Ũ������ȡ�������������������������ͭ�۷�Ӧ��ȡ��������ˮ![]() ��װ������ͼ��ʾ��
��װ������ͼ��ʾ��
![]()
�ش��������⣺
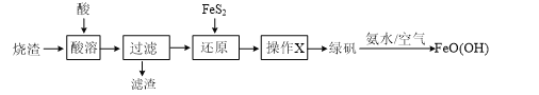
��1��д����A�з�����Ӧ�Ļ�ѧ����ʽΪ��_______________________________________________
��2��B��ѡ�õ��Լ���______________����������________________________��C��ѡ�õ��Լ���______________����������________________________��E��ѡ�õ��Լ���_____________����������________________________��
��3��D�з�Ӧ�Ļ�ѧ����ʽ��________________________________________________
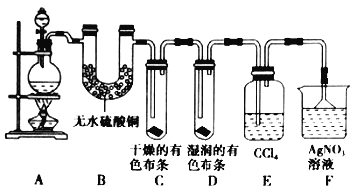
������ijУ��ѧʵ����ȤС��Ϊ��̽����ʵ�����Ʊ�![]() �Ĺ�������ˮ������
�Ĺ�������ˮ������![]() �ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ�������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⡣
�ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ�������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⡣
��4�����ú���![]() ��Ũ������������
��Ũ������������![]() ��Ӧ��
��Ӧ��![]() ���Ƶõ�
���Ƶõ�![]() �������״���£�����С��
�������״���£�����С��![]() ��ԭ����____________________________________________________��
��ԭ����____________________________________________________��
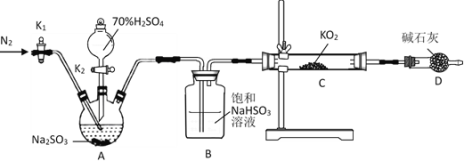
��5����װ��B��������_______________________________________________��������_______________________________________________��
��װ��C��D���ֵIJ�ͬ����˵����������_______________________________________________��
��װ��E��������_______________________________________________��
��6����ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ�����ȷ������ͨ��![]() ��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��
��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��![]() ��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮���ټ�һ��װ�á�����Ϊ��װ��Ӧ����_________��_________֮�䣨��װ����ĸ��ţ���װ����Ӧ����____________________________________________��
��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮���ټ�һ��װ�á�����Ϊ��װ��Ӧ����_________��_________֮�䣨��װ����ĸ��ţ���װ����Ӧ����____________________________________________��
���𰸡�MnO2+4HCl(Ũ)![]() MnCl2+Cl2��+ H2O ����ʳ��ˮ ��ȥHCl���� Ũ���� �������� ����������Һ ���ն����Cl2 Cl2+Cu
MnCl2+Cl2��+ H2O ����ʳ��ˮ ��ȥHCl���� Ũ���� �������� ����������Һ ���ն����Cl2 Cl2+Cu![]() CuCl2 ���ŷ�Ӧ�Ľ��У������Ũ����ϡ�������ٷ�����Ӧ ������ˮ�����ӷ���������������ˮ����������Ժ���̽������������ʵ��������� ��ˮ����ͭ���� ����û��Ư���ԣ���Ư���Ե��Ǵ����� �������� E F ʢ��ʪ��ĵ��۵⻯����ֽ����ʪ�����ɫ������װ��
CuCl2 ���ŷ�Ӧ�Ľ��У������Ũ����ϡ�������ٷ�����Ӧ ������ˮ�����ӷ���������������ˮ����������Ժ���̽������������ʵ��������� ��ˮ����ͭ���� ����û��Ư���ԣ���Ư���Ե��Ǵ����� �������� E F ʢ��ʪ��ĵ��۵⻯����ֽ����ʪ�����ɫ������װ��
��������
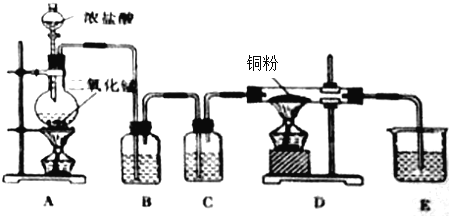
��1��Aװ������ȡCl2����ѧ����ʽΪMnO2+4HCl(Ũ)![]() MnCl2+Cl2��+ H2O��
MnCl2+Cl2��+ H2O��
��2������ʳ��ˮ��ȥHCl��Cװ�õ������Ǹ���Cl2���õ���Ũ���ᣬ�������dz�ȥCl2�е�ˮ������Eװ�������ն����Cl2��������Լ�������������Һ��
��3��Dװ��Cl2�����۵ķ�Ӧ������ʽ��Cl2+Cu![]() CuCl2��
CuCl2��
��4����������ֻ����Ũ���ᷢ����Ӧ����ϡ�����Ӧ���ú���0.2mol HCl��Ũ������������MnO2��Ӧ��ȡ���������ŷ�Ӧ�Ľ��У������Ũ����ϡ�����ٷ�����Ӧ��
��5������ˮ����ͭ��ˮ�����������ܹ�����ˮ����������Bװ�ü�����ˮ�����ӷ���������������ˮ����������Ժ���̽������������ʵ��������ţ�������û��Ư���ԣ���Ư���Ե��Ǵ����
��6���������Ȼ���ͨ�뵽�������������������Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�Ӧ��װ��E��F֮������һ��ʢ��ʪ��ĵ��۵⻯����ֽ����ʪ�����ɫ������װ�ã����ʪ��ĵ��۵⻯����ֽ����������ʪ�����ɫ��������ɫ������������ȫ���ա�