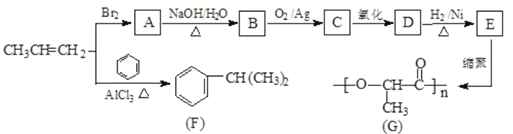
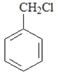
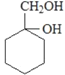
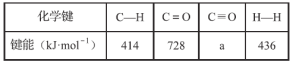
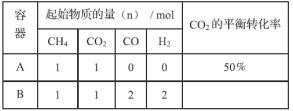
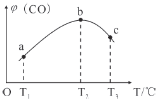
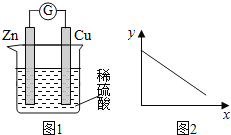
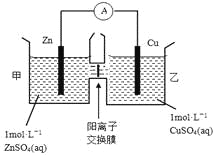
��Ŀ����
����Ŀ��ij������Һ���ⶨ��֪��Ҫ�����Ҵ������л����б�ͪ����������������������±��и����ʵ����ʣ������в�������Ҵ������ᡣ
��֪�����������ڼ���������ˮ�����������ƺ��Ҵ���
���� | ��ͪ | �������� | �Ҵ� | ���� |
�е�/�� | 56.2 | 77.06 | 78.5 | 117.9 |
�����Һ�м����ռ���Һ��������Һ��pH��10���ڽ����Һ�����������л������ȣ����ռ��¶���70��85��ʱ���������ų��������еIJ�Һ����ȴ�������м�Ũ����(����)��Ȼ���ٷ������������н���������������ش��������⣺
(1)�����ռ�ʹ��Һ��pH��10��Ŀ����_______________________��
(2)��70��85��ʱ��������Ҫ�ɷ���________��
(3)�ڲ�����м������Ũ�����Ŀ����(�û�ѧ����ʽ��ʾ)_____________________��
���𰸡�������ת��Ϊ�����ƣ�ʹ���������ڼ���ʱת��Ϊ�����ƺ��Ҵ� �Ҵ� 2CH3COONa��H2SO4��Na2SO4��2CH3COOH
��������
�����ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ����������Ҵ�����Һ����Ҫ����������Һ����ȴ�������м�Ũ���ᣨ����������������ת�������ᣬ�������ռ����ᣬ�Դ˽�ɡ�
(1)�����ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ�������룬�����ᷴӦ���������ƣ�������������Ӧ�����Ҵ��������ƣ���Ӧ����ʽΪ��![]()
![]() ��
��
(2)�������Ϸ������Ҵ��ķе�78�棬������7085��ʱ��������Ҫ�ɷ����Ҵ���
�ʴ�Ϊ���Ҵ���
(3)�����Ϸ������ڲ�����У��������Ũ�����Ŀ���ǽ�������ת�������ᣬ����ʽΪ��![]() ��
��

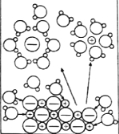
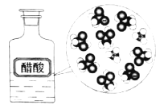
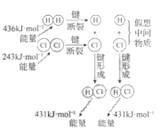
����Ŀ������ʾ��ͼ�뻯ѧ����������ݲ�������ǣ�ˮ����������Ӧ���ӷ��ű�ʾ��
A | B | C | D |
NaCl����ˮ |
���CuCl2��Һ |
CH3COOH��ˮ�е��� |
H2��Cl2��Ӧ�����仯 |
NaCl | CuCl2 | CH3COOH | H2(g)+Cl2(g) ��H=183kJ��mol1 |
A. AB. BC. CD. D