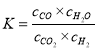��Ŀ����
����Ŀ���ɱ�ϩ�����з�Ӧ�ɵõ�F�߷��ӻ�����G,���Ƕ��dz��õ����ϡ�
����������գ�
(1)F�ķ���ʽΪ___________��E�к��������ŵ������� ___________��
(2)д����Ӧ���ͣ���ϩֱ���γɸ߷���______��A ��B __________��
(3)B ת��Ϊ C �Ļ�ѧ����ʽΪ______________________________��
(4)E��һ��ͬ���칹��M�����������ʣ�
���ܷ���������Ӧ����l molM�������Ľ����Ʒ�Ӧ�ɲ���l molH2
��M�Ľṹ��ʽΪ ______________________��
(5)д����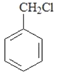 �ϳ�
�ϳ�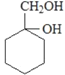 ___________________���ϳ�·�߳��ñ�ʾ����Ϊ��X
___________________���ϳ�·�߳��ñ�ʾ����Ϊ��X![]() Y......
Y......![]() Ŀ����
Ŀ����
���𰸡�C9H12 �Ȼ����ǻ� �Ӿ۷�Ӧ ȡ����Ӧ CH3CH(OH)CH2OH +O2![]() CH3COCHO +2H2O HOCH2CH(OH)CHO
CH3COCHO +2H2O HOCH2CH(OH)CHO 
��������
��ϩ���巢���ӳɷ�Ӧ������A��AΪCH3CHBrCH2Br��A����������ˮ��Һ�����������·���ȡ����Ӧ����B��BΪCH3CH(OH)CH2(OH)��B���������������£�����������������������C��CΪ![]() ��C���ű���������D��DΪ
��C���ű���������D��DΪ![]() ��D�����������£���������ԭ����E��EΪCH3CH(OH)COOH��E�������۷�Ӧ����G����ϩ���Ȼ����������£��뱽������Ӧ����F
��D�����������£���������ԭ����E��EΪCH3CH(OH)COOH��E�������۷�Ӧ����G����ϩ���Ȼ����������£��뱽������Ӧ����F![]() ���ݴ˽��
���ݴ˽��
��1��F�ĽṹʽΪ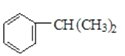 ������9��Cԭ�ӡ�12��Hԭ�ӣ������ʽΪC9H12��E�ĽṹʽΪCH3CH(OH)COOH�����������ŵ��������Ȼ����ǻ����ʴ�Ϊ��C9H12 ���Ȼ����ǻ���
������9��Cԭ�ӡ�12��Hԭ�ӣ������ʽΪC9H12��E�ĽṹʽΪCH3CH(OH)COOH�����������ŵ��������Ȼ����ǻ����ʴ�Ϊ��C9H12 ���Ȼ����ǻ���
��2����ϩ�к���̼̼˫���������Ӿ۷�Ӧ��ֱ���γɸ߷��ӻ����AΪCH3CHBrCH2Br��A����������ˮ��Һ����ȡ����Ӧ����B��BΪCH3CH(OH)CH2(OH)���ʴ�Ϊ���Ӿ۷�Ӧ��ȡ����Ӧ��
��3��B���������������£�����������������������C��CΪ![]() ���仯ѧ����ʽΪCH3CH(OH)CH2OH+O2
���仯ѧ����ʽΪCH3CH(OH)CH2OH+O2![]() CH3COCHO +2H2O���ʴ�Ϊ��CH3CH(OH)CH2OH +O2
CH3COCHO +2H2O���ʴ�Ϊ��CH3CH(OH)CH2OH +O2 ![]() CH3COCHO +2H2O��
CH3COCHO +2H2O��
��4��EΪCH3CH(OH)COOH��E��һ��ͬ���칹��M�����ܷ���������Ӧ��M�к���ȩ������l molM�������Ľ����Ʒ�Ӧ�ɲ���l molH2��M�к��������ǻ�����M�Ľṹ��ʽΪHOCH2CH(OH)CHO���ʴ�Ϊ��HOCH2CH(OH)CHO��
��5����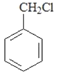 �ϳ�
�ϳ�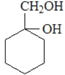 ��������Ϊ��
��������Ϊ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

����Ŀ��������(NaBiO3)�Ƿ�����ѧ�е���Ҫ�Լ�����ˮ�л����ֽ⣬����ˮ������Ѹ�ٷֽ⡣ij��ȤС�����ʵ����ȡ�����Ʋ�̽����Ӧ�á��ش��������⣺
����ȡ������
��ȡװ����ͼ(���Ⱥͼг���������ȥ)�����������������£�
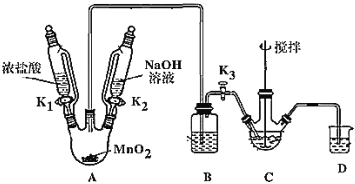
���� | NaBiO3 | Bi(OH)3 |
���� | ��������ˮ��dz��ɫ | ������ˮ����ɫ |
(1)װMnO2������������_____________��Bװ�����ڳ�ȥHCl��ʢ�ŵ��Լ���________________��
(2)C��ʢ��Bi(OH)3��NaOH������Cl2��Ӧ����NaBiO3����Ӧ�����ӷ���ʽΪ_____��
(3)���۲쵽_____(������)ʱ�����Գ����ж� C �з�Ӧ�Ѿ���ɣ�
(4)���װ��ǰ�����ȳ�ȥ��ƿ�в���Cl2������Ⱦ��������ȥCl2�IJ�����_______��
(5)��Ӧ������Ϊ��װ��C�л�þ����ܶ�IJ�Ʒ����Ҫ�IJ�����____________�����ˡ�ϴ�ӡ����
��Ʒ���ȵIJⶨ
(6)ȡ����NaBiO3��Ʒw g����������ϡ�����MnSO4ϡ��Һʹ����ȫ��Ӧ������ c mo1/L��H2C2O4����Һ�ζ����ɵ�MnO4-(��֪��H2C2O4+MnO4---CO2+Mn2++H2O��δ��ƽ)������Һ�Ϻ�ɫǡ����ȥʱ������V mL����Һ���ò�Ʒ�Ĵ���Ϊ ______________(�ú� w��c��V�Ĵ���ʽ��ʾ)��
����Ŀ��ij������Һ���ⶨ��֪��Ҫ�����Ҵ������л����б�ͪ����������������������±��и����ʵ����ʣ������в�������Ҵ������ᡣ
��֪�����������ڼ���������ˮ�����������ƺ��Ҵ���
���� | ��ͪ | �������� | �Ҵ� | ���� |
�е�/�� | 56.2 | 77.06 | 78.5 | 117.9 |
�����Һ�м����ռ���Һ��������Һ��pH��10���ڽ����Һ�����������л������ȣ����ռ��¶���70��85��ʱ���������ų��������еIJ�Һ����ȴ�������м�Ũ����(����)��Ȼ���ٷ������������н���������������ش��������⣺
(1)�����ռ�ʹ��Һ��pH��10��Ŀ����_______________________��
(2)��70��85��ʱ��������Ҫ�ɷ���________��
(3)�ڲ�����м������Ũ�����Ŀ����(�û�ѧ����ʽ��ʾ)_____________________��