��Ŀ����
���ⶨ�����е�SO2��������������ʵ�飺ȡ100 L�ÿ���(��״��)��ͨ��ʢ��100 mL��������(H2O2)ˮ��Һ������ƿ��ʹ�����е�SO2��ȫ���գ��������ᡣ�ڷ�Ӧ���ˮ��Һ�м�������BaCl2��Һ�����ɰ�ɫ���������ⶨ������Ϊ11.65g�������������ɷֶԲⶨ�����Ӱ�죩��
(1)д��SO2��H2O2��Ӧ�����ӷ���ʽ��
(2)д��H2SO4��BaCl2��Ӧ�����ӷ���ʽ��
(3)��100 L������SO2�����������
��1��SO2+H2O2��2H++SO42-��2�֣���2��SO42-+Ba2+��BaSO4����2�֣���3��1.12%��2�֣�
���������������1��SO2���л�ԭ�ԣ�˫��ˮ���������ԣ����߷���������ԭ��Ӧ����Ӧ�����ӷ���ʽ��SO2+H2O2��2H++SO42-��
��2��ϡ������Ȼ�����Ӧ�������ᱵ��ɫ��������Ӧ�����ӷ���ʽ��SO42-+Ba2+��BaSO4����
��3�����ᱵ��������11.65g�����ʵ�����11.65g��233g/mol��0.05mol
��˸���Sԭ���غ��֪��SO2�����ʵ���Ҳ��0.05mol
��100 L������SO2�����������
���㣺����SO2�����ʡ�������SO2�����IJⶨ
�����������ǻ���������Ŀ��飬�ѶȲ����ض�ѧ������֪ʶ�Ĺ��̣�����������ѧ������˼ά�����淶��������������Ĺؼ������ú�ԭ���غ㣬�غ㷨�ǻ�ѧ��������õķ�������Ҫ��ƽʱ��ѧϰ��ע����ۺ��ܽᡢ���ɡ�

ȡһ������Fe��Cu�Ļ�����ĩ��ƽ���ֳ���ȷݣ��ֱ���ÿһ���м���һ������ϡ���ᣬ���跴Ӧ�Ļ�ԭ����ֻ��NO����ʵ�������ɵ�NO�������������ʣ������������¼����(����������ڱ�״���²ⶨ)��
| ʵ����� | 1 | 2 | 3 | 4 | 5 |
| ������Һ��� | 100 mL | 200 mL | 300 mL | 400 mL | 500 mL |
| ʣ���������� | 17.2 g | 8 .0g | 0 g | 0 g | 0 g |
| ������� | 2.24 L | 4.48 L | 6.72 L | 7.84 L | 7.84 L |
(2)ÿһ�ȷݵĻ�����ĩ��ͭ��������
þ�����������仯�������������������й㷺��Ӧ�á�
��1��þ���Ͻ����ڷɻ�����ҵ������3��90��þ���Ͻ�����������2mol/Lϡ����������0��2mol���������㲢ȷ��þ���Ͻ������ʵ���n(Mg): n(Al)= ��
��2�����������Ҫ�ɷ�ΪFeS2(��������ֻ��SiO2)�����������ԭ�ϡ�ȡij������10g�������Ŀ��������գ�4FeS2+11O2��2Fe2O3+8SO2������ַ�Ӧ����ȴ���Ƶù�������Ϊ7��4g������SiO2����Ӧ��������������FeS2����������Ϊ ��
��3������һ���������ۺ�������ɵĻ�����100 mLϡ�����ַ�Ӧ����Ӧ���������κ�����ų�������Ӧ��ij�����Һ������4��00 mol��L��1��NaOH��Һ������NaOH��Һ���������������������Ĺ�ϵ��ͼ��ʾ(��Ҫʱ�ɼ��ȣ�����������ˮ�е��ܽ�)����������A�����ֵ�� ��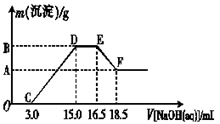
��4���������������������������֡�ȡij������ĩ28��12g(����ֻ��Fe��C)�����������г�ַ�Ӧ���õ�CO2����224mL(��״����)��
�ټ���˸�����ĩ������̼�����ʵ���֮��Ϊ ����������ȣ���
����ȡ���ݲ�ͬ����������������ĩ�ֱ�ӵ�100mL��ͬŨ�ȵ�ϡH2SO4�У���ַ�Ӧ��õ�ʵ���������±���ʾ��
| ʵ����� | �� | �� | �� |
| ���������ĩ��������g�� | 2��812 | 5��624 | 8��436 |
| ��������������L������״���� | 1��120 | 2��240 | 2��800 |
���������Һ�����ʵ���Ũ��Ϊ ��
����������ʵ����м�������m�˸�����ĩ�����㷴Ӧ������ʣ��Ĺ�������Ϊ g (����3λС��)
�ڶ�����»ᡰ�Ҹ�֮�š���������Ͻ�������졣���Ͻ�����
| A���� | B�������� | C�������� | D������� |