��Ŀ����
ʵ��������CuSO4��5H2O������480mL0.1mol/L��CuSO4��Һ����ش��������⣺
��1��Ӧ��������ƽ��ȡCuSO4��5H2O����_______g��
��2�����ڳ�����Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ�1g����ʹ�����룩����ƽƽ��ʱ��ʵ�ʳ�����CuSO4��5H2O������__________g��
��3����ʵ���õ�����Ҫ�����У�������ƽ����Ͳ���ձ�����������________��________��
��4�����ƹ������м����ؼ��IJ���Ͳ�������ͼ��ʾ������Щʵ�鲽��A��F��ʵ������Ⱥ������
�� ��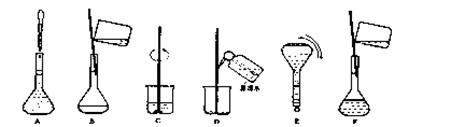
���ж��ݵľ�������� ��
��5�����������ʹ������Һ��Ũ�Ȳ�������Ӱ�죨A.ƫ�� B.ƫ�� C.����,����š�����
���ܽ⾧���õ��ձ��Ͳ�����δϴ�ӣ�____________��
�ڶ���ʱ���ӿ̶��ߣ�____________��
������CuSO4��5H2O������ʧȥ���ֽᾧˮ��____________��
��1��12.5 ��2��11.5 ��3��500ml����ƿ ��ͷ�ι�
��4��CBDFAE ���ж��ݵľ��������:������ƿ�м�������ˮ����Һ����̶���1��2cm��ʱ�����ý�ͷ�ιܼ�����ˮ����Һ����͵���̶������� ��5��B��A��A
���������������1��������Һ�����Ϊ480ml��������ƿ�Ĺ��û��480ml��ֻ��ѡ��500ml����ƿ�������ҪCuSO4�����ʵ���n��cV��0.5L��0.1mol?L-1��0.05mol��CuSO4?5H2O�����ʵ�������CuSO4�����ʵ���������CuSO4?5H2O��������0.05mol��250g/mol��12.5g��
��2����ҩƷ������ŷ���������ƽƽ��ԭ��m(����)��m(����)+�����������ʵ�ʳ�������ͭ���������Ϊ12g-0.5g��11.5g��
��3����Һ����һ�㲽���ǣ�������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��һ������ƽ�������õ�ҩ�ף����壬���ձ����ܽ⣬���ò��������裬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȡ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ���˻���Ҫ������Ϊ��500mL����ƿ��ҩ�ס���ͷ�ιܡ�
��4���ɣ�3������Һ���Ƶ�һ�㲽���֪����ȷ�IJ���˳��Ϊ��CBDFAE�����ж��ݵľ�������ǣ�������ƿ�м�������ˮ����Һ����̶���1��2cm��ʱ�����ý�ͷ�ιܼ�����ˮ����Һ����͵���̶������С�
��5�����ܽ⾧���õ��ձ��Ͳ�����δϴ�ӣ���������ͭմ���ձ����벣�����ϣ���������ƿ������ͭ�����ʵ������٣�������Һ��Ũ��ƫ�ͣ��ʴ�Ϊ��B��
�ڶ���ʱ���ӿ̶��ߣ�Һ���ڿ̶����·�����Һ�����ƫС��������Һ��Ũ��ƫ�ߣ��ʴ�Ϊ��A��
������CuSO4?5H2O������ʧȥ���ֽᾧˮ������ͭ��������������ʵ�ʳ���������ͭ����������ͭ������ƫ��������Һ��Ũ��ƫ����ѡA��
���㣺��������ͭ��Һ�����ơ�����ѡ��������Ȼ���ʵ������Լ���������

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�ʵ����Ҫ��98%���ܶ�Ϊ1��84g��cm-3������������3��68mol/L��������Һ480mL��
��1����ȷ��ȡ98%������ mL��
��2��Ҫ����3��68mol/L��������Һ480mL�������õ����������ձ�������������Ͳ
��3��������3��68mol/L��������Һ��������������ȷ�����������д����������ʹ�����Ƶ�������ҺŨ��ƫ�͵��� ��
| A����ϡ�͵�������Һת��������ƿ��δϴ���ձ��Ͳ����� |
| B�����ձ��ڵ�ϡ����������ƿ��ת��ʱ�����������ʹ����ϡ���ὦ��ƿ�� |
| C���ý�ͷ�ι�������ƿ�м�ˮʱ��Һ���������ƿ�̶ȣ���ʱ�����õιܽ�ƿ��Һ��������ʹ��Һ��Һ����̶����� |
| D���ý�ͷ�ι�������ƿ�м�ˮʱ�����ӹ۲���Һ��Һ��������ƿ�̶����� |
ijһ��θҩ�е������Ϊ̼���,��������������������IJⶨ����:
��������0.1 mol��L-1��HCl��Һ��0.1 mol��L-1��NaOH��Һ
��ȡһ��(ҩƬ������ͬ)0.2 g�Ĵ�θҩƬ,ĥ������20.0 mL����ˮ
���Է�̪Ϊָʾ��,��0.1 mol��L-1��NaOH��Һ�ζ�,��ȥV mL��ζ��յ�
�ܼ���25 mL 0.1 mol��L-1��HCl��Һ
(1)д��ʵ����̵IJ���(д���˳��)������������������������
(2)��ͼ��ʾ����������0.1 mol��L-1��HCl��Һ��0.1 mol��L-1��NaOH��Һ�϶�����Ҫ��������(�����)������,����������Һ����Ҫ�IJ���������������(����������)�� 
(3)����������ҺӦѡ�õ�����ƿ�����(����ĸ)������������
| A��50 mL��50 mL |
| B��100 mL��100 mL |
| C��100 mL��150 mL |
| D��250 mL��250 mL |
(5)ÿ��θҩ�к�̼��Ƶ�����Ϊ������ g��
��ͼ�������Լ�ƿ��ǩ�ϵ����ݡ�
| �����ѧ��(CP)(500mL) Ʒ�������� ��ѧʽ��H2SO4 ��Է���������98 �ܶȣ�1.84g��cm-3 ����������98% |
��2��ʵ�����ø���������240mL 0.46mol/L��ϡ���ᣬ��
�������������� A.�ձ� B.100mL��Ͳ C.250mL����ƿ D.500mL����ƿ E.������ F.������ƽ�������룩G.10mL��ͲH.��ͷ�ιܣ�����ʱ������ʹ�õ������� ������ţ�.
����Ҫ����������Ϊ mL.
����ͼΪ���ƹ����еļ����ؼ�����Ͳ�����������ʵ�鲽��A��F��ʵ�����
�Ⱥ�������� ��
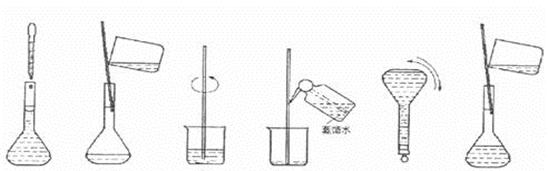
|
�ܸ�ͬѧʵ�����Ƶõ���Ũ��Ϊ0.45mol/L�����ܵ�ԭ����
A����ȡŨH2SO4ʱ���ӿ̶�
B������ƿϴ����δ�����ﴦ��
C��û�н�ϴ��Һת������ƿ
D������ʱ���ӿ̶�
�¹�������ҵ��־��Ӧ�û�ѧ���Ͽ������½��ܣ�ij�е������о�С�����������һ�֡�ˮƿ�����ø���ϩ(C60)��������������ƿ�塱��һ������������ƿ�ǡ���ǡ�ÿɽ�һ��ˮ���ӹ������档����˵����ȷ���� ( )
| A��ˮ��˫��ˮ��ˮ�������Ǵ����� |
| B��ʯī��C60����Ϊͬλ�� |
| C��������ǿ�����ǿ����� |
| D��һ��������ʯīת��ΪC60�ǻ�ѧ�仯 |
���з���������ȷ����
��Һ�ȡ���ˮ���ɱ����⻯����Ϊ������
��CaCl2��NaOH��HCl��IBr��������
��������ˮ�����ռ�����Ϊǿ�����
��C60��C70�����ʯ��ʯī��Ϊ̼��ͬ��������
�ݵ�ơ����ۡ�ˮ�������ײ��Ͼ�Ϊ����
| A���٢ۢܡ����� | B���ڢۡ��� | C���ڢܡ��� | D���ڢۢܢ� |
���෨�ڻ�ѧѧ�Ƶķ�չ������Ҫ���á����з������������
| A�����ݷ�ɢϵ���ȶ��Դ�С�����Ϊ��Һ���������Һ |
| B�����ݷ�Ӧ����ЧӦ����ѧ��Ӧ��Ϊ���ȷ�Ӧ�����ȷ�Ӧ |
| C������������к��е�Hԭ�Ӹ��������ΪһԪ�ᡢ��Ԫ��Ͷ�Ԫ�� |
| D������Ԫ��ԭ�������������Ķ��ٽ�Ԫ�ط�Ϊ����Ԫ�غͷǽ���Ԫ�� |