��Ŀ����
1��Na2SO4•10H2O����â����ҽ���Ͽ�����к�ȣ�������ᣬ��ʵ�Ȼ��ͣ����ͱ��أ�̵ͣ���ۣ�������1.0mol•L-1��Na2SO4QUOTE QUOTE��Һ100mL��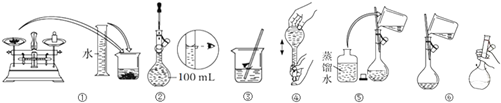
��1��ijͬѧ�ù���Na2SO4•10H2O��������Na2SO4��Һ�IJ�����ͼ��ʾ��������ù�����ȷ��˳���ǣ���д��ţ��٢ۢޢݢڢܣ�
��2���������в�����������Һ��Ũ�Ȳ�����Ӱ�죬���������գ�
��Na2SO4•10H2O����ʧȥ�˲��ֽᾧˮ
��ת����Һ���ձ��Ͳ�����δ����ϴ��
�۶���ʱ���ӿ̶���
��[����ҡ�Ⱥ�Һ���½����ּ�ˮ���̶���
������ƿδ������ʹ����������������ҺŨ��ƫ�ߵ��Т٢ۣ�����ţ���ͬ����ƫ�͵��Тڢܣ�
��3����������������SO42-�������μ��������BaCl2��Na2CO3�����ˡ�����Һ�м�ϡ���ᣬ�����ᾧ��NaCl���壮
���� ��1���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ�����������
��2������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��3�������е�SO42-�������μ��������BaCl2��̼��������ȥ��
��� �⣺��1���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��ȷ�IJ���˳��Ϊ�٢ۢޢݢڢܣ��ʴ�Ϊ���٢ۢޢݢڢܣ�
��2����Na2SO4•10H2O����ʧȥ�˲��ֽᾧˮ���������ҩƷ������Na2SO4������ƫ�ߣ������Ƴ���Ũ��ƫ�ߣ�
��ת����Һ���ձ��Ͳ�����δ����ϴ�ӣ����������ʵ���ʧ��Ũ��ƫ�ͣ�
�۶���ʱ���ӿ̶��ߣ�����Һ���ƫС��Ũ��ƫ�ߣ�
�ܶ���ҡ�Ⱥ�Һ���½��������ģ��ּ�ˮ���̶��ߣ���Ũ��ƫ�ͣ�
������ƿδ���������ҺŨ����Ӱ�죮
�ʴ�Ϊ���٢ۣ��ڢܣ�
��3�������е�SO42-�����ȼ��������BaCl2������ȥSO42-��Ȼ�����̼��������ȥ�����ı����ӣ�����������ȥ������̼������ʴ�Ϊ��BaCl2��Na2CO3��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д����������м������ϡH2SO4�����ˣ�
������Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��������MnSO4���Ϻ�ɫ��ʧ�����ˣ�
����Ũ�����ᾧ�����룬�õ���Ʒ��
��1��H2SO4�ܽ�A12O3�����ӷ���ʽ��Al2O3+6H+=2Al3++3H2O
��2��KMnO4����Fe2+�����ӷ���ʽ����������
1MnO4+5Fe2++8H+=1Mn2++5Fe3++4H2O
��3����֪��
�����������������pH
| Al��OH��3 | Fe��OH��2 | Fe��OH��3 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
���ݱ������ݽ��Ͳ�����Ŀ�ģ���ȥ��Ԫ��
��4����֪��һ�������£�MnO4-����Mn2+��Ӧ����MnO2��
�����ij����м���ŨHCI�����ȣ���˵�������д���MnO2��������Ũ������MnO2�������ɻ���ɫ����Cl2�����ж�MnO2�Ƿ���ڣ�
�ڢ��м���MnSO4��Ŀ���dz�ȥ������MnO4-��
| A�� | �Ȼ����Ħ����������NA���ȷ��Ӻ�NA������ӵ�����֮�� | |
| B�� | ���³�ѹ�£�11.2 L�����к�����ԭ����ΪNA | |
| C�� | ��������Ϊ0.5molMgCl2����Һ�к���Cl-������ΪNA | |
| D�� | 32 g�����к�����ԭ����ΪNA |
| A�� | �ڴ�����Һ�м�������NaHSO4���壬����ĵ���ƽ�����ƣ�����Һ�е�c��H+����С | |
| B�� | ����ˮ���ȵ�100�棬���PH��7��˵�������¶ȿ�ʹˮ������ | |
| C�� | 0.2mol/L��������ˮ�������ϣ�������Һ��PH=1 | |
| D�� | PH=3��ϡ������PH=11�İ�ˮ�������ϣ�������Һ��PH=7 |
| A�� | ��������ʱ������ʹ������е�ˮ����ȫ���ɺ��ֹͣ���� | |
| B�� | �������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ��֧�ܿڴ� | |
| C�� | ��Һ����ʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� | |
| D�� | ����һ�����ʵ���Ũ����Һת�Ʋ���ʱ�������¶˵�������ƿ�̶��ߴ� |
| A�� | ��ϩ�����ʽ��ʵ��ʽ��Ϊ��C2H4 | B�� | �Ҵ��Ľṹ��ʽΪ��C2H6O | ||
| C�� | ȩ���ļ�д��ʽΪ��-CHO | D�� | �������м��е�������˫�� |
 ����������ԭ��Ӧ��2Ag+��aq��+Zn��s���TZn2+��aq��+2Ag��s����Ƶ�ԭ�����ͼ��ʾ����ش��������⣺
����������ԭ��Ӧ��2Ag+��aq��+Zn��s���TZn2+��aq��+2Ag��s����Ƶ�ԭ�����ͼ��ʾ����ش��������⣺