��Ŀ����
��������к���Cr(OH)3��Al2O3��ZnO��CuO��NiO������,��ҵ��ͨ�������±��ա���������������Na2Cr2O7�����ʡ�
��֪:��Na2CrO4��Һ�к�������NaAlO2��Na2ZnO2������
(1)ˮ�������Һ������������(��ᡱ��������С�)��
(2)����������չ���������Na2CrO4�Ļ�ѧ����ʽ��
��������Cr(OH)3+��������Na2CO3+������������ ��������Na2CrO4+��������CO2+������������
��������Na2CrO4+��������CO2+������������
(3)���������Ҫ�ɷ���Zn(OH)2������������
(4)��ϵ�в�������Ϊ:��������H2SO4,��������,��ȴ�ᾧ,���ˡ���������H2SO4Ŀ������ ��
��֪:�ٳ�ȥ����II��,��Һ�д������·�Ӧ:
2CrO42��+2H+ Cr2O72��+H2O
Cr2O72��+H2O
��Na2Cr2O7��Na2CrO4�ڲ�ͬ�¶��µ��ܽ�����±�
| �¶� �ܽ�� ��ѧʽ | 20 �� | 60 �� | 100 �� |
| Na2SO4 | 19.5 | 45.3 | 42.5 |
| Na2Cr2O7 | 183 | 269 | 415 |
| Na2CrO4 | 84 | 115 | 126 |
(1)��
(2)4Cr(OH)3+4Na2CO3+3O2 4Na2CrO4+4CO2+6H2O
4Na2CrO4+4CO2+6H2O
(3)Al(OH)3
(4)����Ũ�����ٽ�ƽ��2CrO42��+2H+ Cr2O72��+H2O������Ӧ�����ƶ�,���������ɸ����ܽ�ȸ����Na2Cr2O7
Cr2O72��+H2O������Ӧ�����ƶ�,���������ɸ����ܽ�ȸ����Na2Cr2O7
(5)CrO42��+8H++6e- Cr+4H2O
Cr+4H2O
����

����ѧ��ѡ��2����ѧ�뼼������15�֣�
��̬��ҵ���Ľ��裬�����������ֻ��������Ҫ����ѭ���������ۺͳ�ֿ��Ǿ��õĿɳ�����չ��������ij��ҵ��Ƶ����ᣭ��泥�ˮ����������ˮ����ˮ���ã��Σ��ȣ�������������̬��ҵ������ͼ��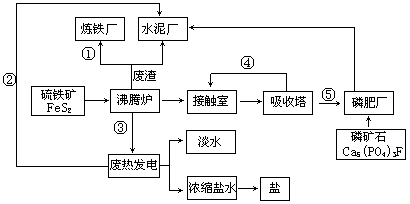
����������ҵ���̻ش��������⣺
��1����ԭ�ϡ���Դ����ͨ�Ƕȿ��Ǹ���ҵӦ���ڣ�������
����
| A������ɽ������ | B���غ��������� | C��������С��� | D��������½ |
��3������¯������Ӧ�Ļ�ѧ����ʽ�������������������������������� ,�ʳ��IJ�Ʒ���ոƣ�����Ҫ�ɷ��������������� (�ѧʽ)��
��4���ȵ糧����ȴˮ��������������������Ũ����ˮ����ȡ���������ȡ��������������д��һ�ּ��ɣ���
��5��������̬�����������������¯�������������������������ܵ��������������������������������������������������������� ����д�����㣩��
����ĽṹΪCH3��CH2��COOH,�������ǰ�ȫ��Ч�ķ�ù��������,һ���Լ�ʽ̼��пΪԭ�ϵ�����������������: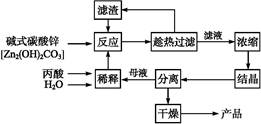
| ��� | n(����)�� n(��ʽ̼��п) | ��Ӧ�¶�/�� | ����п����/% |
| 1 | 1��0.25 | 60 | 67.2 |
| 2 | 1��0.25 | 80 | 83.5 |
| 3 | 1��0.25 | 100 | 81.4 |
| 4 | 1��0.31 | 60 | 89.2 |
| 5 | 1��0.31 | 80 | 90.1 |
| 6 | 1��0.31 | 100 | 88.8 |
(1)̽����ʵ������ѹ�������(���ϱ�):��Ӧʱ��2 h,��ˮ��45 g,n(����)��n(��ʽ̼��п)=1��
 ,��Ӧ�¶����������档
,��Ӧ�¶����������档 (2)�����ղ��á���·ѭ������ʽ,�������Ʊ����ռ�㡢���ʸ���,�������������������������������������������ŵ㡣
(3)ij��ʵ��ʱ,��37.0 g��������220 mLˮ��,�����������������Ż����������Ʊ�,���յñ���п49.6 g,��ô�ʵ�����п�IJ���Ϊ��������(д���������)��
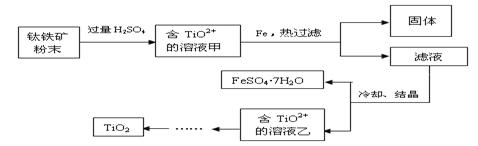
�����ѣ�Ti������Ӳ�ȴ��۵�ߡ�����ʱ����ʴ�����㷺�������¿Ƽ����ϣ�����Ϊ��δ��������������������Ҫ�ɷ�FeTiO3������������Ϊ��Ҫԭ��ұ��������ͬʱ��ø���Ʒ�Ĺ�ҵ�����������£��ش��������⣺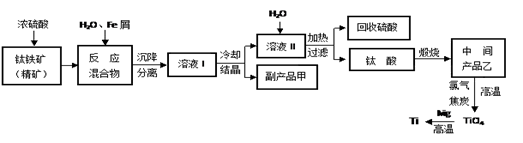
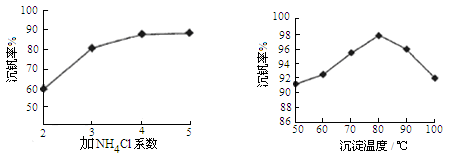
��1���������Ũ���ᷴӦ�IJ���֮һ��TiOSO4����Ӧ�����������ɡ�����Ʒ���׳ơ��̷����仯ѧʽ��________________��
��2���������������м���Feм��Ŀ���� �������ӷ���ʽ��ʾ�������鸱��Ʒ���Ƿ���ʵ�ʵ�鷽���� ��
��3�������������������õ��Ľ������л����������ʣ��ɼ��� �ܽ���ȥ��
��4����Һ���к���Fe2+��TiO2+������Mg2+�������ӡ������£����Ӧ���������Ksp���±���ʾ��
| �������� | Fe��OH)2 | TiO(OH)2 | Mg(OH)2 |
| Ksp | 8.0��10-16 | 1.0��10-29 | 1.8��10-11 |
����������Fe2+��TiO2+��Mg2+����Һ��ˮϡ�ͣ���������������ɫ������д���÷�Ӧ�����ӷ���ʽ��__________________________________________________________________
��5��Mg��ԭTiCl4�����б�����1070K���¶��½��У�����Ϊ��Ӧ���Ƶķ�Ӧ������__________________________
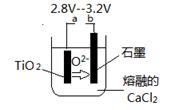
��6����800--1000��ʱ���TiO2Ҳ���Ƶú����ѣ�װ����ͼ��ʾ��ͼ��b�ǵ�Դ��______���������ĵ缫��Ӧʽ________________��
����ĽṹΪCH3��CH2��COOH,�������ǰ�ȫ��Ч�ķ�ù��������,һ���Լ�ʽ̼��пΪԭ�ϵ�����������������: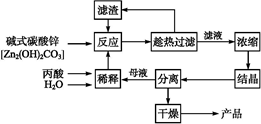
| ��� | n(����)�� n(��ʽ̼��п) | ��Ӧ�¶�/�� | ����п����/% |
| 1 | 1��0.25 | 60 | 67.2 |
| 2 | 1��0.25 | 80 | 83.5 |
| 3 | 1��0.25 | 100 | 81.4 |
| 4 | 1��0.31 | 60 | 89.2 |
| 5 | 1��0.31 | 80 | 90.1 |
| 6 | 1��0.31 | 100 | 88.8 |
(1)̽����ʵ������ѹ�������(���ϱ�):��Ӧʱ��2 h,��ˮ��45 g,n(����)��n(��ʽ̼��п)=1��
 ,��Ӧ�¶����������档
,��Ӧ�¶����������档 (2)�����ղ��á���·ѭ������ʽ,�������Ʊ����ռ�㡢���ʸ���,���������������������������ŵ㡣
(3)ij��ʵ��ʱ,��37.0 g��������220 mLˮ��,�����������������Ż����������Ʊ�,���յñ���п49.6 g,��ô�ʵ�����п�IJ���Ϊ��������(д���������)��
�������ʵ��Ʊ������Ϲ�ҵ����ʵ�ʵ���( )
| A��������ͨ�����ʯ��ˮ����Ư�� |
| B�������ӽ���Ĥ����ⱥ��ʳ��ˮ�Ʊ��ռ���������� |
| C����������������Ϻ��ȼ��������ˮ�����Ʊ����� |
| D����SO2��O2�Ļ�����Ӹ�ѹ��ͨ���Ӵ��ң��Ʊ�SO3 |