��Ŀ����
�������ѹ㷺Ӧ���ڸ���ṹ����Ϳ�ϡ�ֽ��Ϳ��ȣ��������ѻ�����Ϊ�Ʊ��ѵ��ʵ�ԭ�ϡ�
�������ѿ����������ַ����Ʊ���
����1��TiCl4ˮ������TiO2��xH2O�����ˡ�ˮϴ��ȥ���е�Cl�����ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2���˷����Ʊ��õ��������������ѡ�
��1���� TiCl4ˮ������TiO2��x H2O�Ļ�ѧ����ʽΪ_______________________________��
�� ����TiO2��x H2O��Cl���Ƿ����ķ�����______________________________��
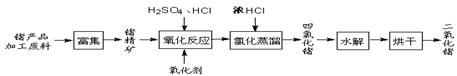
����2�����ú���Fe2O3����������Ҫ�ɷ�ΪFeTiO3������TiԪ�ػ��ϼ�Ϊ+4�ۣ���ȡ������Ҫ�������£�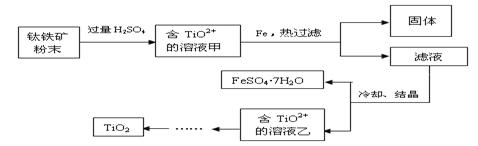
��2��Fe2O3��H2SO4��Ӧ�����ӷ���ʽ�� ��
��3������Һ�г���TiO2+֮����еĽ����������� ��
��4����Fe�������� ��
��.�������ѿ�������ȡ�ѵ���
��5��TiO2��ȡ����Ti���漰���IJ������£�
��Ӧ�ڵķ���ʽ�� ���÷�Ӧ��Ҫ��Ar�����н��У������ԭ��_____________��
��1����TiCl4+��x+2��H2O==TiO2?xH2O��+4HCl��ȡ���һ��ϴ��Һ���������Թ��У��μ������ữ��AgNO3��Һ����������ɫ������˵��Cl-�ѳ���
��2��Fe2O3+6H+=2Fe3++3H2O
��3��Fe3+��Fe2+��
��4����Fe3+ת��ΪFe2+
��5��TiCl4+2Mg=2MgCl2+Ti�� ��ֹ������Mg��Ti��������е�O2����CO2��N2�����ã�
���������������1������TiCl4��ϵ��Ϊ1������Ԫ���غ㣬TiO2?xH2O��ϵ��Ϊ1��HCl��ϵ��Ϊ4���ٸ���OԪ���غ㣬��֪H2O��ϵ��Ϊ��2+x��������ʽΪTiCl4+��x+2��H2O?TiO2?xH2O��+4HCl���ڳ���������Һ�е�Cl-�����ݷ�����Ӧ��Cl-+Ag+�TAgCl����ȡ����ϴ��Һ��������Һ���ܽ�������Ƿ��ڣ�
��2�������������ᷴӦ������������ˮ�����ӷ���ʽΪ��Fe2O3+6H+=2Fe3++3H2O��
��3��Fe2O3+6H+=2Fe3++3H2O��FeTiO3+4H+=Fe2++TiO2++2H2O�����Ի����ڵ���������Fe3+��Fe2+
��4������Һ�к��������ӣ������л�ԭ�ԣ��ܽ������������������������Ҳ������µ����ʣ��������������ǣ���Fe3+ת��ΪFe2+
��5����800�������£����Ȼ��Ѻ�þ��Ӧ�����Ȼ�þ���ѣ���Ӧ����ʽΪ��TiCl4+2Mg==2MgCl2+Ti ��Mg�ǻ��ý�������������ж������ʷ�Ӧ����˿ɵó�Ar������Ϊ����������ֹMg�Ϳ��������ʷ�Ӧ
���㣺����ѧ���Թ����������⡢�Ķ���Ŀ��ȡ��Ϣ������������ԭ��Ӧ������ʽ����д�ȣ��Ѷ��еȣ�����������ǹؼ�����Ҫѧ�������Ķ���Ŀ��ȡ��Ϣ������������û���֪ʶ�������⡢�������������

������˵������ȷ����____ ��
| A����������ijЩ���ַ����������� |
| B����������Ӳ�ȵ�ˮ��Ҫ�ü��ȵķ������������� |
| C�����Ṥҵ�У��ڽӴ��Ұ�װ�Ƚ�������Ϊ������S03ת��ΪH2S04ʱ�ų������� |
| D���ϳɰ���ҵԭ��������ʱ������̼�����Һ���ճ�ȥ������̼ |
��ش��������⣺
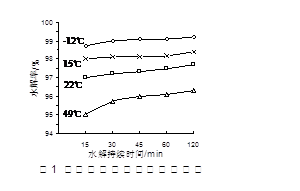
��1�������ӽ���Ĥ��ⱥ��ʳ��ˮʱ�����Ƶı���ʳ��ˮӦ�ü��뵽 ���ҡ�
��2����֪�����ӽ���Ĥ�����У�������ÿСʱͨ��1�����ֱ���磬ÿ�ۿ��Բ���1��492 g���ռij������300�����۴�������8Сʱ���Ƶ�32%���ռ���Һ���ܶ�Ϊ1��342��103 kg/m3��113 m3�����۵ĵ���ǿ��1��45��l04 A���õ��۵ĵ��Ч��Ϊ ��
��3��ʾ��ͼ����ȡNaHC03�Ļ�ѧ����ʽΪ ��
��4���������ֱ�Ӽ���Mg��OH��2�õ�Mg0���ٵ������Mg0�ý���Mg�������ɼ����̡����жϸ÷����Ƿ���У���˵������ ��
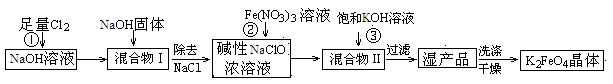
��������к���Cr(OH)3��Al2O3��ZnO��CuO��NiO������,��ҵ��ͨ�������±��ա���������������Na2Cr2O7�����ʡ�
��֪:��Na2CrO4��Һ�к�������NaAlO2��Na2ZnO2������
(1)ˮ�������Һ������������(��ᡱ��������С�)��
(2)����������չ���������Na2CrO4�Ļ�ѧ����ʽ��
��������Cr(OH)3+��������Na2CO3+������������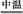 ��������Na2CrO4+��������CO2+������������
��������Na2CrO4+��������CO2+������������
(3)���������Ҫ�ɷ���Zn(OH)2������������
(4)��ϵ�в�������Ϊ:��������H2SO4,��������,��ȴ�ᾧ,���ˡ���������H2SO4Ŀ������ ��
��֪:�ٳ�ȥ����II��,��Һ�д������·�Ӧ:
2CrO42��+2H+ Cr2O72��+H2O
Cr2O72��+H2O
��Na2Cr2O7��Na2CrO4�ڲ�ͬ�¶��µ��ܽ�����±�
| �¶� �ܽ�� ��ѧʽ | 20 �� | 60 �� | 100 �� |
| Na2SO4 | 19.5 | 45.3 | 42.5 |
| Na2Cr2O7 | 183 | 269 | 415 |
| Na2CrO4 | 84 | 115 | 126 |
��ҵ�����÷���м(����������������������)������ʽ������[Fe(OH)SO4]�Ĺ����������£�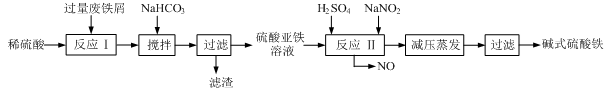
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
�ش��������⣺
��1�������������м��Ŀ���� ����ʱ��Һ�д��ڵ���������Ҫ�� ����NaHCO3������ҺpHʱ�����ӷ���ʽ�� ��
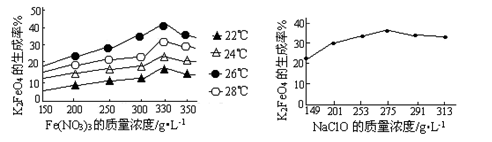
��2����ʵ�������У���Ӧ���г�ͬʱͨ��O2�Լ���NaNO2��������ͬʱͨ��O2�������� ��
��3����ʽ����������ˮ���ܵ������[Fe(OH)]2+���ӣ�д��[Fe(OH)]2+����ˮ�ⷴӦ����Fe(OH)3�����ӷ���ʽ ��
��4����֪����м����Ԫ�ص���������Ϊ84.0%����������ÿ����Ӧ����Ԫ�ص���ģ�����100�ַ���м��������������� �ּ�ʽ��������
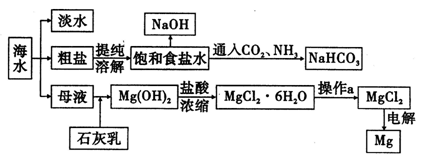
��ͼ��ʾ��һ���ۺϴ���SO2�����Ĺ������̣���ÿ������ȫ��Ӧ������˵����ȷ���� (����)
| A����ҺB�з����ķ�ӦΪ2SO2��O2=2SO3 |
| B���������Ը��������Һ������ҺC���Ƿ���Fe3�� |
| C�����������̿���֪�����ԣ�Fe3��>O2>SO42�� |
| D���˹��յ��ŵ�֮һ��������ѭ������ |
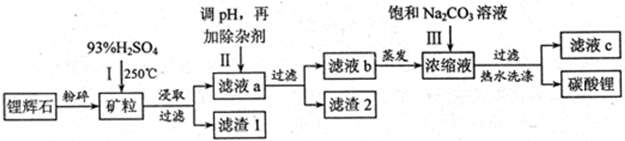
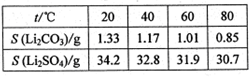
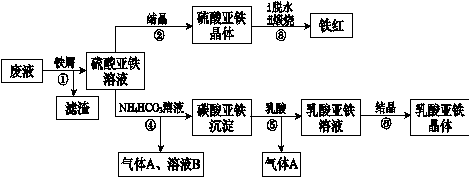
 Li2SO4��Al2O3��4SiO2��H2O��
Li2SO4��Al2O3��4SiO2��H2O��