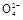��Ŀ����
Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���ˮ��þ����Ҫ��������:
��ش���������:
(1)�����ӷ�Ӧ�ĽǶ�˼��,�ں�ˮ�м���ʯ�������������������,д���ڳ������з�����Ӧ�����ӷ���ʽ����������������������������
(2)ʯ��������ʯ����ˮ�γɵĻ�����,�ӳ�����ú���ѧ��Դ,��߾���Ч��ĽǶ�,������ʯ�ҵ���Ҫԭ����Դ�ں����е�����������
(3)����A����������,����B������������
(4)����������Լ�a����������(�ѧʽ)��
(5)��ˮMgCl2������״̬��,ͨ�������Mg��Cl2,�÷�Ӧ�Ļ�ѧ����ʽΪ���������������ӿ��dzɱ��ͷ���ѭ�����õĽǶ�,����������������������������
(6)��ˮ��þ�Ĺ���,ΪʲôҪ����ˮ�е��Ȼ�þת��Ϊ������þ,��ת��Ϊ�Ȼ�þ?
_____________________________________________
(1)����Mg2+[��ʹMg2+�γ�Mg(OH)2����](1��)
Mg2++2OH- Mg(OH)2��(1��)
Mg(OH)2��(1��)
(2)����(��ĵ�ÿǵ�)(1��)
(3)���ˡ������ᾧ(�����Ũ��)(ÿ��0.5��)
(4)����(1��)
(5)MgCl2(����) Mg+Cl2��(2��)��������,ѭ��ʹ��(1��)
Mg+Cl2��(2��)��������,ѭ��ʹ��(1��)
(6)��ˮ���Ȼ�þ�ĺ����ܴ�,��þ����Ũ�Ⱥܵ�,�ù��̿���ʹþ���Ӹ���,Ũ�������ҳɱ���(2��)
����

 ��У����ϵ�д�
��У����ϵ�д������й�˵����ȷ����
| A�����¼�������þ��̼�Ļ��������Ƶ���þ |
| B������ұ��������ͨ���û���Ӧ�õ������� |
| C����ˮ����Ĺ����в�����������ԭ��Ӧ |
| D�����õ��ķ������ԴӺ�ˮ�л�õ�ˮ |
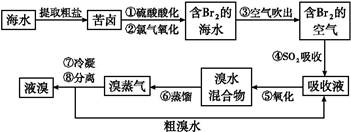
��ˮ��þ���ܴ���ԼΪ2.1��1015t��Ŀǰ������60%��þ���Ժ�ˮ����ҵ��ģ��ˮ��þ��������ͼ��ʾ��
�Իش��������⣺
(1)��ˮ��þ�Ĺ����Т١��ڷ�Ӧ�Ļ�ѧ����ʽ��
��_____________________________________��
��______________________________________��
(2)���Ȼ�þ��Һ��ȡ��ˮ�Ȼ�þ���壬�����������____________________
(3)Ϊ��ʹMgSO4��ȫת��ΪMg(OH)2����������ʯ��Ҫ������Ȼ������Mg(OH)2����������Ca(OH)2�ܽ�ȣ�Ӧ����________�����롣
(4)����þ�����Ĺ�ҵұ��������������֮�������в�֮ͬ���±�������þ���Ȼ�þ���۷е����ݣ�
| ���� | ����þ | �Ȼ�þ |
| �۵�(��) | 2 852 | 714 |
| �е�(��) | 3 600 | 1 412 |
��ҵ����þ���õ�������Ȼ�þ����ұ�������õ�����ڵ�Al2O3����ԭ����____________________________________
������˵������ȷ����____ ��
| A����������ijЩ���ַ����������� |
| B����������Ӳ�ȵ�ˮ��Ҫ�ü��ȵķ������������� |
| C�����Ṥҵ�У��ڽӴ��Ұ�װ�Ƚ�������Ϊ������S03ת��ΪH2S04ʱ�ų������� |
| D���ϳɰ���ҵԭ��������ʱ������̼�����Һ���ճ�ȥ������̼ |
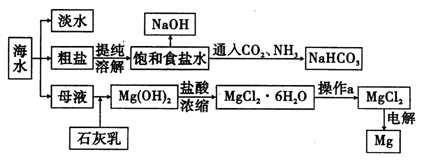
��ش��������⣺
��1�������ӽ���Ĥ��ⱥ��ʳ��ˮʱ�����Ƶı���ʳ��ˮӦ�ü��뵽 ���ҡ�
��2����֪�����ӽ���Ĥ�����У�������ÿСʱͨ��1�����ֱ���磬ÿ�ۿ��Բ���1��492 g���ռij������300�����۴�������8Сʱ���Ƶ�32%���ռ���Һ���ܶ�Ϊ1��342��103 kg/m3��113 m3�����۵ĵ���ǿ��1��45��l04 A���õ��۵ĵ��Ч��Ϊ ��
��3��ʾ��ͼ����ȡNaHC03�Ļ�ѧ����ʽΪ ��
��4���������ֱ�Ӽ���Mg��OH��2�õ�Mg0���ٵ������Mg0�ý���Mg�������ɼ����̡����жϸ÷����Ƿ���У���˵������ ��
��������к���Cr(OH)3��Al2O3��ZnO��CuO��NiO������,��ҵ��ͨ�������±��ա���������������Na2Cr2O7�����ʡ�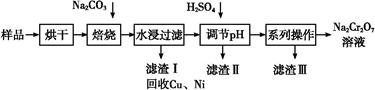
��֪:��Na2CrO4��Һ�к�������NaAlO2��Na2ZnO2������
(1)ˮ�������Һ������������(��ᡱ��������С�)��
(2)����������չ���������Na2CrO4�Ļ�ѧ����ʽ��
��������Cr(OH)3+��������Na2CO3+������������ ��������Na2CrO4+��������CO2+������������
��������Na2CrO4+��������CO2+������������
(3)���������Ҫ�ɷ���Zn(OH)2������������
(4)��ϵ�в�������Ϊ:��������H2SO4,��������,��ȴ�ᾧ,���ˡ���������H2SO4Ŀ������ ��
��֪:�ٳ�ȥ����II��,��Һ�д������·�Ӧ:
2CrO42��+2H+ Cr2O72��+H2O
Cr2O72��+H2O
��Na2Cr2O7��Na2CrO4�ڲ�ͬ�¶��µ��ܽ�����±�
| �¶� �ܽ�� ��ѧʽ | 20 �� | 60 �� | 100 �� |
| Na2SO4 | 19.5 | 45.3 | 42.5 |
| Na2Cr2O7 | 183 | 269 | 415 |
| Na2CrO4 | 84 | 115 | 126 |

 2H++2Br-+S
2H++2Br-+S