��Ŀ����
����ĽṹΪCH3��CH2��COOH,�������ǰ�ȫ��Ч�ķ�ù��������,һ���Լ�ʽ̼��пΪԭ�ϵ�����������������: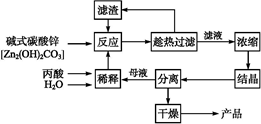
| ��� | n(����)�� n(��ʽ̼��п) | ��Ӧ�¶�/�� | ����п����/% |
| 1 | 1��0.25 | 60 | 67.2 |
| 2 | 1��0.25 | 80 | 83.5 |
| 3 | 1��0.25 | 100 | 81.4 |
| 4 | 1��0.31 | 60 | 89.2 |
| 5 | 1��0.31 | 80 | 90.1 |
| 6 | 1��0.31 | 100 | 88.8 |
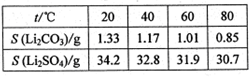
(1)̽����ʵ������ѹ�������(���ϱ�):��Ӧʱ��2 h,��ˮ��45 g,n(����)��n(��ʽ̼��п)=1��
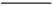 ,��Ӧ�¶����������档
,��Ӧ�¶����������档 (2)�����ղ��á���·ѭ������ʽ,�������Ʊ����ռ�㡢���ʸ���,���������������������������ŵ㡣
(3)ij��ʵ��ʱ,��37.0 g��������220 mLˮ��,�����������������Ż����������Ʊ�,���յñ���п49.6 g,��ô�ʵ�����п�IJ���Ϊ��������(д���������)��
(1)0.31��80��(3)ԭ�������ʸ�,��Һ�������ŷŵ�
(3)n(����)= ="0.5" mol;n(����п)=
="0.5" mol;n(����п)= ="0.235" mol;����=
="0.235" mol;����= ��100%=94.0%
��100%=94.0%
����

��ˮ��þ���ܴ���ԼΪ2.1��1015t��Ŀǰ������60%��þ���Ժ�ˮ����ҵ��ģ��ˮ��þ��������ͼ��ʾ��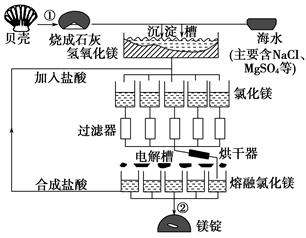
�Իش��������⣺
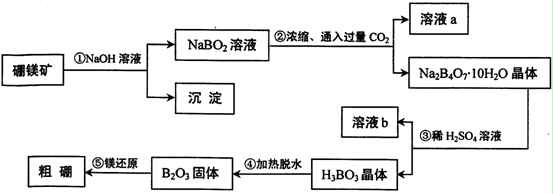
(1)��ˮ��þ�Ĺ����Т١��ڷ�Ӧ�Ļ�ѧ����ʽ��
��_____________________________________��
��______________________________________��
(2)���Ȼ�þ��Һ��ȡ��ˮ�Ȼ�þ���壬�����������____________________
(3)Ϊ��ʹMgSO4��ȫת��ΪMg(OH)2����������ʯ��Ҫ������Ȼ������Mg(OH)2����������Ca(OH)2�ܽ�ȣ�Ӧ����________�����롣
(4)����þ�����Ĺ�ҵұ��������������֮�������в�֮ͬ���±�������þ���Ȼ�þ���۷е����ݣ�
| ���� | ����þ | �Ȼ�þ |
| �۵�(��) | 2 852 | 714 |
| �е�(��) | 3 600 | 1 412 |
��ҵ����þ���õ�������Ȼ�þ����ұ�������õ�����ڵ�Al2O3����ԭ����____________________________________
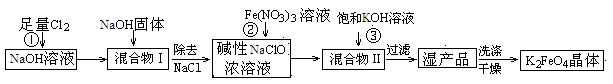
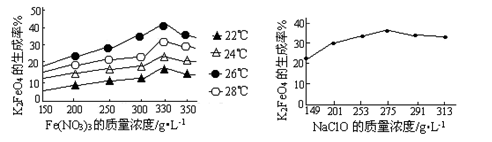
��������к���Cr(OH)3��Al2O3��ZnO��CuO��NiO������,��ҵ��ͨ�������±��ա���������������Na2Cr2O7�����ʡ�
��֪:��Na2CrO4��Һ�к�������NaAlO2��Na2ZnO2������
(1)ˮ�������Һ������������(��ᡱ��������С�)��
(2)����������չ���������Na2CrO4�Ļ�ѧ����ʽ��
��������Cr(OH)3+��������Na2CO3+������������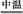 ��������Na2CrO4+��������CO2+������������
��������Na2CrO4+��������CO2+������������
(3)���������Ҫ�ɷ���Zn(OH)2������������
(4)��ϵ�в�������Ϊ:��������H2SO4,��������,��ȴ�ᾧ,���ˡ���������H2SO4Ŀ������ ��
��֪:�ٳ�ȥ����II��,��Һ�д������·�Ӧ:
2CrO42��+2H+ Cr2O72��+H2O
Cr2O72��+H2O
��Na2Cr2O7��Na2CrO4�ڲ�ͬ�¶��µ��ܽ�����±�
| �¶� �ܽ�� ��ѧʽ | 20 �� | 60 �� | 100 �� |
| Na2SO4 | 19.5 | 45.3 | 42.5 |
| Na2Cr2O7 | 183 | 269 | 415 |
| Na2CrO4 | 84 | 115 | 126 |
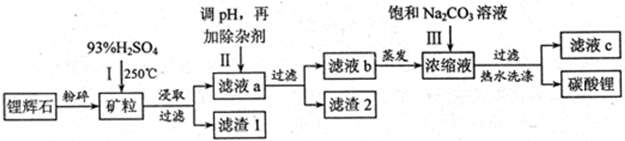
��ҵ�����÷���м(����������������������)������ʽ������[Fe(OH)SO4]�Ĺ����������£�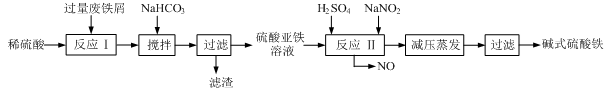
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
�ش��������⣺
��1�������������м��Ŀ���� ����ʱ��Һ�д��ڵ���������Ҫ�� ����NaHCO3������ҺpHʱ�����ӷ���ʽ�� ��
��2����ʵ�������У���Ӧ���г�ͬʱͨ��O2�Լ���NaNO2��������ͬʱͨ��O2�������� ��
��3����ʽ����������ˮ���ܵ������[Fe(OH)]2+���ӣ�д��[Fe(OH)]2+����ˮ�ⷴӦ����Fe(OH)3�����ӷ���ʽ ��
��4����֪����м����Ԫ�ص���������Ϊ84.0%����������ÿ����Ӧ����Ԫ�ص���ģ�����100�ַ���м��������������� �ּ�ʽ��������
�����ǵ�һ���˹��ϳɵ��л�����й������ص���������ȷ���� (����)��
| A��������һ�ֵ��� |
| B�������������³´�л��һ�ֲ��� |
| C�������ܷ���ˮ�ⷴӦ |
| D��������һ���������� |
 2I��+S4O62����
2I��+S4O62����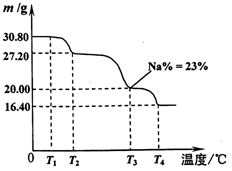
 Li2SO4��Al2O3��4SiO2��H2O��
Li2SO4��Al2O3��4SiO2��H2O��