��Ŀ����
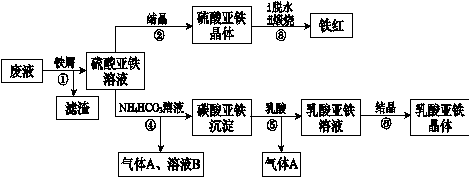
�����ѣ�Ti������Ӳ�ȴ��۵�ߡ�����ʱ����ʴ�����㷺�������¿Ƽ����ϣ�����Ϊ��δ��������������������Ҫ�ɷ�FeTiO3������������Ϊ��Ҫԭ��ұ��������ͬʱ��ø���Ʒ�Ĺ�ҵ�����������£��ش��������⣺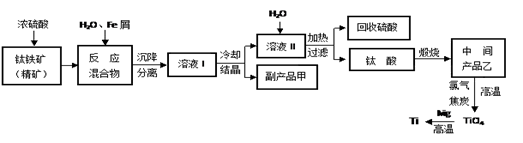
��1���������Ũ���ᷴӦ�IJ���֮һ��TiOSO4����Ӧ�����������ɡ�����Ʒ���׳ơ��̷����仯ѧʽ��________________��
��2���������������м���Feм��Ŀ���� �������ӷ���ʽ��ʾ�������鸱��Ʒ���Ƿ���ʵ�ʵ�鷽���� ��
��3�������������������õ��Ľ������л����������ʣ��ɼ��� �ܽ���ȥ��
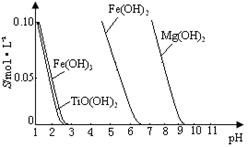
��4����Һ���к���Fe2+��TiO2+������Mg2+�������ӡ������£����Ӧ���������Ksp���±���ʾ��
| �������� | Fe��OH)2 | TiO(OH)2 | Mg(OH)2 |
| Ksp | 8.0��10-16 | 1.0��10-29 | 1.8��10-11 |
����������Fe2+��TiO2+��Mg2+����Һ��ˮϡ�ͣ���������������ɫ������д���÷�Ӧ�����ӷ���ʽ��__________________________________________________________________
��5��Mg��ԭTiCl4�����б�����1070K���¶��½��У�����Ϊ��Ӧ���Ƶķ�Ӧ������__________________________
��6����800--1000��ʱ���TiO2Ҳ���Ƶú����ѣ�װ����ͼ��ʾ��ͼ��b�ǵ�Դ��______���������ĵ缫��Ӧʽ________________��
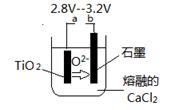
�� FeSO4��7H2O �� 2Fe3��+Fe=3Fe2�� ȡ����Ʒ����������ˮ���μ�����KSCN��Һ���۲���Һ�Ƿ��ΪѪ��ɫ �� ϡ���� ��4����10 ��TiO2++2H2O=TiO(OH)2��+2H+ ��5���������������ڶ��������Χ�У����з�Ӧ�� ��6����TiO2 +4e-= Ti��2O2-
��������������� �̷��Ļ�ѧʽ��FeSO4��7H2O���� �������������м���Feм��Ŀ���Ƿ�ֹ��������������Ӧ�����ӷ���ʽ��2Fe3��+Fe=3Fe2�� �����鸱��Ʒ���Ƿ���ʵ�ʵ�鷽����ȡ����Ʒ����������ˮ���μ�����KSCN��Һ���۲���Һ�Ƿ��ΪѪ��ɫ������ΪѪ��ɫ֤�������ˣ�����δ���ʡ��� �����������������õ��Ľ������л�����������Mg���ɸ���þ�Ļ�Ա���ǿ���������ᷢ����Ӧ��ͨ�������������ܽ��ȥ���� Ksp=C(Mg2+)C2(OH-)=1.8��10-11;����ʼ������������=C(Mg2+)C2(OH-)> Ksp�� C2(OH-)=(1.8��10-11)��C(Mg2+)=(1.8c)��0.0018mol/L=1.��10-8,����C(OH-)=1��10-4.C(H+)=11��10-10.��PH="10." ����������Fe2+��TiO2+��Mg2+����Һ��ˮϡ�ͣ���������������ɫ�������÷�Ӧ�����ӷ���ʽ��TiO2++2H2O=TiO(OH)2��+2H+ ����5��Mg��ԭTiCl4�����б�����1070K���¶��½��У����ڸ�����Mg����������Ti���ױ����������ʣ��������ܲ���Ti�����Ի�Ӧ���Ƶķ�Ӧ�����Ǹ������������ڶ��������Χ�У����з�Ӧ�� ��6����TiO2 +4e-= Ti��2O2-��6��TiO2��������ʯī��������a�Ǹ�����b�������������ĵ缫��Ӧʽ��TiO2 +4e-= Ti��2O2-��
���㣺����Ti��ұ����������ȡ�������漰�ĸ����ʵĻ�ѧ���ʡ����ӵļ����֪ʶ��

(1)ij����ÿ��Ҫ�յ�����1.6%����ú200 t,�ŷų���SO2��������Ⱦ����,������Ϊ��,����ЩSO2��������,��ô������ÿ��(��365 d��)������98%��Ũ������������;
(2)��Ҫ�����Ƽ��������,����Ӧ���Դ����������������,���Һ��������������Һ,��ƹ�������������Һ��Ũ�Ȼ���������(�������С�����䡱);
(3)��ҵ������ˮ�ࡢ����ʱ��Ҫ�õ���ԭ������������(����),����ѡԭ���Ʋ����Ļ�ѧ����ʽ���� ��;
| A������ | B����ʯ�� | C��ʯ��ʯ | D����� |
(5)��������ˮ��ԭ����
(�����ӷ���ʽ��ʾ);������ʱӲ�ȵ�ˮ�г�ȥMg2+�ķ������� ��
�� (�û�ѧ����ʽ��ʾ)��
��������к���Cr(OH)3��Al2O3��ZnO��CuO��NiO������,��ҵ��ͨ�������±��ա���������������Na2Cr2O7�����ʡ�
��֪:��Na2CrO4��Һ�к�������NaAlO2��Na2ZnO2������
(1)ˮ�������Һ������������(��ᡱ��������С�)��
(2)����������չ���������Na2CrO4�Ļ�ѧ����ʽ��
��������Cr(OH)3+��������Na2CO3+������������ ��������Na2CrO4+��������CO2+������������
��������Na2CrO4+��������CO2+������������
(3)���������Ҫ�ɷ���Zn(OH)2������������
(4)��ϵ�в�������Ϊ:��������H2SO4,��������,��ȴ�ᾧ,���ˡ���������H2SO4Ŀ������ ��
��֪:�ٳ�ȥ����II��,��Һ�д������·�Ӧ:
2CrO42��+2H+ Cr2O72��+H2O
Cr2O72��+H2O
��Na2Cr2O7��Na2CrO4�ڲ�ͬ�¶��µ��ܽ�����±�
| �¶� �ܽ�� ��ѧʽ | 20 �� | 60 �� | 100 �� |
| Na2SO4 | 19.5 | 45.3 | 42.5 |
| Na2Cr2O7 | 183 | 269 | 415 |
| Na2CrO4 | 84 | 115 | 126 |
��ҵ�����÷���м(����������������������)������ʽ������[Fe(OH)SO4]�Ĺ����������£�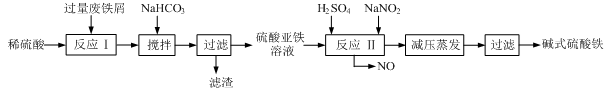
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
�ش��������⣺
��1�������������м��Ŀ���� ����ʱ��Һ�д��ڵ���������Ҫ�� ����NaHCO3������ҺpHʱ�����ӷ���ʽ�� ��
��2����ʵ�������У���Ӧ���г�ͬʱͨ��O2�Լ���NaNO2��������ͬʱͨ��O2�������� ��
��3����ʽ����������ˮ���ܵ������[Fe(OH)]2+���ӣ�д��[Fe(OH)]2+����ˮ�ⷴӦ����Fe(OH)3�����ӷ���ʽ ��
��4����֪����м����Ԫ�ص���������Ϊ84.0%����������ÿ����Ӧ����Ԫ�ص���ģ�����100�ַ���м��������������� �ּ�ʽ��������
�ֽ���������ϲ��ϵĻ�����(����)��
| A��ˮ�� | B��ɳ�� |
| C���ֽ� | D�������� |