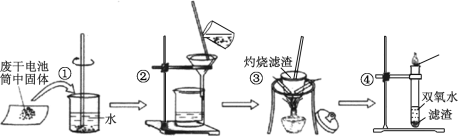
��Ŀ����
����Ŀ��������̼����������Ӧ������仯����Ľ������֮һ���ش��������⣺
(l)�ҹ������Ŷ����õ��µ�������Эͬ���������ڳ��³�ѹ��ʵ���˽�CO2��CH4һ��ת��Ϊ������Ʒ����д�� CO2��CH4�ϳ�������Ȼ�ѧ����ʽ��____��
������������ȼ���ȷֱ�Ϊ-890.31 kJ/mol��-876.72 kJ/mol��
(2)��ijһ�����ܱ�������CH4��CO2�ķ�ѹ�ֱ�Ϊ15 kPa��20 kPa������Ni/��-Al2 O3������������1123 Kʹ�䷢����Ӧ��CH4(g)+CO2(g)![]() 2CO(g)+2H2(g)��
2CO(g)+2H2(g)��
�������CO����������v��CO��=1.28![]() 10-2�qp��CH4��
10-2�qp��CH4��![]() p��CO2����kPa
p��CO2����kPa![]() s-1����ijʱ�̲��p(H2)=10 kPa���� p��CH4��=___kPa��v��CO��=___kPa
s-1����ijʱ�̲��p(H2)=10 kPa���� p��CH4��=___kPa��v��CO��=___kPa![]() s-1��
s-1��
�ڴﵽƽ�������ϵѹǿ����ʼʱ��![]() ����÷�Ӧ��ƽ�ⳣ��Kp=____kPa��2��
����÷�Ӧ��ƽ�ⳣ��Kp=____kPa��2��
(3)������(GaN)��Cu�������ͼ��ʾ���˹����ϵͳ����װ������CO2��H2OΪԭ�Ϻϳ�CH4��
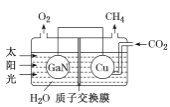
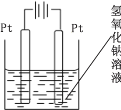
�ٸ�װ�ù���ʱH+����____���GaN����Cu�����缫���õ缫�ϵĵ缫��ӦʽΪ ___��
�ڸ�װ��ÿ����1 mol CH4��������Һ��������____g��
�۱�ʵ�������£���CO2ת��Ϊ��������顢��ϩ�ȣ���ת����Ϊ10%������CH4��ѡ����Ϊ12%�����ռ���12 mol CH4����ͨ���CO2Ϊ____mol������֪��ѡ����=����Ŀ��������ĵ�ԭ������ԭ���ܵ�ת������
(4)�����˹����ϵͳװ��Ҳ�����Ʊ���ϩ����Ȳ����Ҫ����ԭ�ϡ�2010��Sheth���о��ó���Ȳ��Pd����ѡ�����ķ�Ӧ����������ͼ��ʾ������������Pd����������á�*����ע��
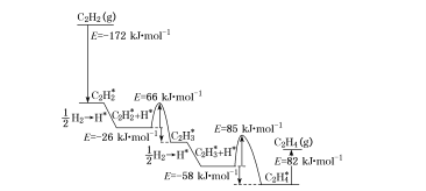
����������ӦΪ ____����ȡ����ȡ�����Ӧ���ù�������С���ݣ���ܣ�Ϊ___ kJ![]() mol-1���ò���Ļ�ѧ����ʽΪ____��
mol-1���ò���Ļ�ѧ����ʽΪ____��
���𰸡�CH4��g��+CO2��g����CH3COOH��l����H=-13.59 kJ/mol 10 1.92 3200 Cu CO2+8e-+8H+=CH4+2H2O 72 1000 ���� 66 C2H2*+H*=C2H3*
��������
��1�����ݼ���������ȼ���ȵó��Ȼ�ѧ����ʽΪ���� CH4��g��+2O2��g����CO2��g��+2H2O��l�� ��H=-890.31 kJ/mol���� CH3COOH��l��+2O2��g��=2CO2��g��+2H2O��l�� ��H=-876.72 kJ/mol �����ݸ�˹���ɢ�-�ڵ�CH4��g��+CO2��g���TCH3COOH��l����H=-890.31 kJ/mol-(-876.72 kJ/mol)=-13.59 kJ/mol����CO2��CH4�ϳ�������Ȼ�ѧ����ʽΪ��CH4��g��+CO2��g����CH3COOH��l����H=-13.59 kJ/mol���ʴ�Ϊ��CH4��g��+CO2��g����CH3COOH��l����H=-13.59 kJ/mol��
��2������ijһ�����ܱ�������CH4��CO2�ķ�ѹ�ֱ�Ϊ15 kPa��20 kPa������Ni/��-A12 O3������������1123 Kʹ�䷢����Ӧ��CH4(g)+CO2(g)![]() 2CO(g)+2H2(g)����
2CO(g)+2H2(g)����

p��CH4��=10kPa��v��CO��=1.28![]() 10-2 p��CH4��
10-2 p��CH4��![]() p��CO2����kPa
p��CO2����kPa![]() s-1��=1.28
s-1��=1.28![]() 10-2��10��15=1.92��kPa
10-2��10��15=1.92��kPa![]() s-1�����ʴ�Ϊ��10��1.92��
s-1�����ʴ�Ϊ��10��1.92��
�ڴﵽƽ�������ϵѹǿ����ʼʱ��![]() ����ﵽƽ��״̬���ļ����ѹx��
����ﵽƽ��״̬���ļ����ѹx��

15-x+20-x+2x+2x=��15+2����![]() �����x=10�����ƽ�ⳣ��Kp=
�����x=10�����ƽ�ⳣ��Kp=![]() ���ʴ�Ϊ��3200��
���ʴ�Ϊ��3200��
��3������ͼ��֪��������ˮʧȥ�������������������϶�����̼�õ��������ɼ��飬�������������ƶ��� �����ĵ缫��ӦΪ��CO2+8e-+8H+=CH4+2H2O���ʴ�Ϊ��Cu��CO2+8e-+8H+=CH4+2H2O��
�ڸ���������Ӧʽ֪ÿ����1mol CH4ת�Ƶ�����Ϊ8mol��������Һ�������ٵ���ˮ�����������ݵ���ת���غ㼰�缫��ӦʽH2O-4e-=O2��+4H+�ã�m(H2O)=18g/mol��![]() =72g���ʴ�Ϊ��72��
=72g���ʴ�Ϊ��72��
���������n��CO2����10%��12%=12mol����n��CO2��=1000mol���ʴ�Ϊ��1000��
��4������ͼʾ֪��������Ӧ�������ͣ���ӦΪ���ȣ�����ͼʾ��Ӧ����С���ݣ���ܣ�Ϊ66 kJ![]() mol-1���ò���Ļ�ѧ����ʽΪ��C2H2*+H*=C2H3*���ʴ�Ϊ�����ȣ�66��C2H2*+H*=C2H3*��
mol-1���ò���Ļ�ѧ����ʽΪ��C2H2*+H*=C2H3*���ʴ�Ϊ�����ȣ�66��C2H2*+H*=C2H3*��

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�����Ŀ��п����Ԫ���γɵĻ������ڸ�����������Ҫ�����á�
(1)��̬Zn2+�ļ۵����Ų�ʽΪ_______________��
(2)������NH3�����У�H-N-H������Ϊ107��18������ͼ��[Zn(NH3)6]2���IJ��ֽṹ�Լ�����H-N-H�����ǡ�
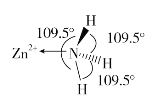
�����[Zn(NH3)6]2��������H-N-H���DZ�Ϊ109.5����ԭ����_____________��
(3)����Һ����кܸߵ�Ӧ�ü�ֵ������EMIM��������H��C��N����Ԫ����ɣ���ṹ��ͼ��ʾ��
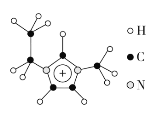
���������÷�����![]() ��ʾ������m��n�ֱ���������γɴ�������ԭ�����͵���������EMIM�������еĴ�����Ӧ��ʾΪ___________________��������[EMIM][AlCl4]���кܸߵ�Ӧ�ü�ֵ�����۵�ֻ��7 �棬�����ʾ����������________��
��ʾ������m��n�ֱ���������γɴ�������ԭ�����͵���������EMIM�������еĴ�����Ӧ��ʾΪ___________________��������[EMIM][AlCl4]���кܸߵ�Ӧ�ü�ֵ�����۵�ֻ��7 �棬�����ʾ����������________��
(4)����Ԫ��ˮ�������Ƿ�����ɫ��ԭ�ӽṹ�йأ��Ҵ���һ���Ĺ��ɣ���֪Zn2���ȹ���Ԫ�������γɵ�ˮ�����ӵ���ɫ���±���ʾ��
���� | Sc3�� | Cr3�� | Fe2�� | Zn2+ |
ˮ�����ӵ���ɫ | ��ɫ | ��ɫ | dz��ɫ | ��ɫ |
�����ԭ�ӽṹ�Ʋ�Sc3����Zn2����ˮ������Ϊ��ɫ��ԭ��____________________��
(5)Zn��S�γ�ij�ֻ�����ľ�����ͼ��ʾ��
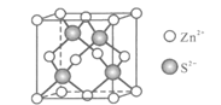
��Zn2+����S2����ɵ�________________��϶�У�
���ɢ��ܷ��жϳ�S2����Zn2+���У�_________������������������������֪�����ܶ�Ϊdg/cm3��S2���뾶Ϊa pm����ҪʹS2-��Zn2+���У���Zn2+�뾶Ϊ____________________pm��д�������ʽ����