��Ŀ����
13������������Ҫ�Ĺ������ϣ��÷���м�Ʊ������������£��ش��������⣺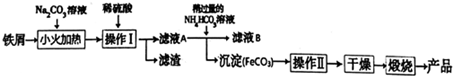
��1���ټ�̼������Һ��С����ȵIJ���Ŀ����ȥ�����ۣ�
�ڲ�����������ǹ��ˣ��������������ϴ�ӣ�
��2���������������ķ�ӦΪFeSO4+2NH4HCO3=FeCO3��+��NH4��2SO4+CO2��+H2O�����У������Թ�����NH4HCO3��Һ����Ҫ������Һ��Ϊ6.8��7.2֮�䣮�����Թ�����Ŀ����ʹ��Һ������������ȫת��ΪFeCO3��������Һ��pH���ܹ��͵�ԭ����̼����������ᷴӦ��̼�������������٣�
�ڼ�����ҺB�к���NH4+�ķ�����ȡ����B��Һ���Թ��У���������Ũ����������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����ҺB�к���笠����ӣ�
��3����Щͬѧ��ΪKMnO4��Һ�ζ��ܽ�����Ԫ�غ����IJⶨ��
a����ȡ2.85g�̷���FeSO4•7H2O����Ʒ�����250mL��Һ��
b����ȡ25.00mL������Һ����ƿ�У�
c�������ữ��0.0100mol/L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
��д������KMnO4��Һ��FeSO4��Һ��Ӧ�����ӷ���ʽ5Fe2++MnO4-+8H+=Fe3++2Mn2++4H2O��
����KMnO4����Һ�ζ�ʱӦѡ����ʽ�ζ��ܣ����ʽ����ʽ������
�ۼ���������Ʒ��FeSO4•7H2O����������Ϊ97.54%[��֪M��FeSO4•7H2O��=278g/mol]��
���� ̼������Һ�Լ��ԣ����Գ�ȥ��м��������ۣ���ϡ�����ܽ�������ͨ�����˷���õ���ҺA����ҺA�м���̼����泥�����Һ����������ת��ΪFeCO3��������ͨ�����˷�����ҺB��FeCO3����ҺB�к�������泥�FeCO3���渽�����ʣ��ڸ���ǰӦ��ϴ�ӳ�ȥ���ʣ��ڿ��������շֽ⣬��+2�����ڿ����м����ױ�����Ϊ+3�ۣ����յõ�Fe2O3��
��1����̼������Һ�Լ��ԣ����Գ�ȥ��м��������ۣ�
�ڲ���I��õ���������Һ��ӦΪ���ˣ���������ǰӦ��ϴ�ӳ�ȥ���ʣ�
��2���ټ����ٹ�����̼����泥�ʹ��Һ������������ȫת��ΪFeCO3������pH���ͣ�̼����������ᷴӦ��̼�������������٣�
������笠�������Ӧ���ɰ�����������ʹʪ��ĺ�ɫʯ����ֽ������
��3���ٸ����������ǿ�����ԣ��������Ӿ��л�ԭ�ԣ����������£����������������������Ϊ�����ӣ���������ԭΪ�����ӣ�ͬʱ��ˮ���ɣ�
�ڸ��������Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ�Ӧʢ������ʽ�ζ����У�
�۸���n=cV����25mL��Ʒ��Һ���ĸ�����ص����ʵ�������������250mL��Һ�������ĸ�����ص����ʵ������ٸ��ݢ������ӷ���ʽ�����������ӵ����ʵ�������FeԪ���غ�ɵ�FeSO4•7H2O�����ʵ���������m=nM����FeSO4•7H2O����������������������������
��� �⣺̼������Һ�Լ��ԣ����Գ�ȥ��м��������ۣ���ϡ�����ܽ�������ͨ�����˷���õ���ҺA����ҺA�м���̼����泥�����Һ����������ת��ΪFeCO3��������ͨ�����˷�����ҺB��FeCO3����ҺB�к�������泥�FeCO3���渽�����ʣ��ڸ���ǰӦ��ϴ�ӳ�ȥ���ʣ��ڿ��������շֽ⣬��+2�����ڿ����м����ױ�����Ϊ+3�ۣ����յõ�Fe2O3��
��1����̼������Һ�Լ��ԣ��ٽ�����ˮ�⣬���Գ�ȥ��м��������ۣ��ʴ�Ϊ��ȥ�����ۣ�
�ڲ���I���뻥�����ܵĹ�����Һ�壬��ȡ���ˣ�̼���������渽�����ʣ���������ǰӦ��ϴ�ӳ�ȥ���ʣ��ʴ�Ϊ�����ˣ�ϴ�ӣ�
��2���ټ����ٹ�����̼����泥�ʹ��Һ������������ȫת��ΪFeCO3������pH���ͣ�̼����������ᷴӦ��̼�������������٣�
�ʴ�Ϊ��ʹ��Һ������������ȫת��ΪFeCO3������̼����������ᷴӦ��̼�������������٣�
�ڼ���笠����ӵ�ʵ�鷽��Ϊ��ȡ����B��Һ���Թ��У���������Ũ����������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����ҺB�к���笠����ӣ�
�ʴ�Ϊ��ȡ����B��Һ���Թ��У���������Ũ����������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����ҺB�к���笠����ӣ�
��3���ٸ����������ǿ�����ԣ��������Ӿ��л�ԭ�ԣ����������£����������������������Ϊ�����ӣ���������ԭΪ�����ӣ�ͬʱ��ˮ���ɣ���Ӧ���ӷ���ʽΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
�ʴ�Ϊ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
�ڸ��������Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ���Ϊ�����ữ��Ӧʢ������ʽ�ζ����У��ʴ�Ϊ����ʽ��
��25mL��Ʒ��Һ���ĸ�����ص����ʵ���Ϊ0.02L��0.0100mol/L=2��10-4mol����֪250mL��Һ�������ĸ�����ص����ʵ���Ϊ2��10-4mol��$\frac{250mL}{25mL}$=2��10-3mol����5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O����֪250mL��Һ�������������ӵ����ʵ���Ϊ2��10-3mol��5=0.01mol����FeԪ���غ�ɵ�FeSO4•7H2O�����ʵ���Ϊ0.01mol����FeSO4•7H2O����������Ϊ$\frac{0.01mol��278g/mol}{2.85g}$��100%=97.54%��
�ʴ�Ϊ��97.54%��
���� ���⿼�黯ѧ�������̣�ע������Լ��ߡ������ߡ���Ӧ�ߣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

��1���л���X ������ͼΪ�� | ��1���л���X����Է���������100�� |
| ��2����10.0g X������O2�г��ȼ�գ���ʹ���������ͨ����������ˮCaCl2��KOHŨ��Һ��������ˮCaCl2����7.2g��KOHŨ��Һ����22.0g�� | ��2���л���X�ķ���ʽ�� C5H8O2�� |
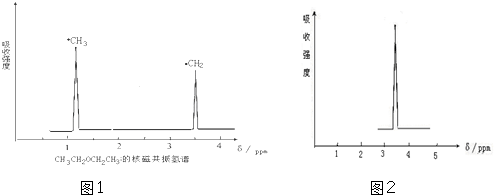
| ��3����������ײⶨ���л���X�к���ȩ�����л���X�ĺ˴Ź�������ͼ����2�����շ壬�����֮����3��1�� | ��3���л���X�Ľṹ��ʽ�� ��CH3��2C��CHO��2�� |

| A�� | װ�â��У������е�K+����ZnSO4��Һ | |
| B�� | װ�âڹ���һ��ʱ���a��������Һ��pH���� | |
| C�� | ��װ�â۾���ͭʱ��c��Ϊ��ͭ | |
| D�� | װ�âܵ�ظ����ĵ缫��ӦʽΪ��O2+4e-+2H2O=4OH- |
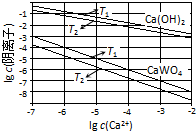 ��֪�������ƺ�����ƣ�CaWO4�������ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С������ұ�������У����������Ƽ��������Ƽ�����Һ�еõ�����ƣ�������Ӧ��WO42-��aq��+Ca��OH��2��s���TCaWO4��s��+2OH-��aq����
��֪�������ƺ�����ƣ�CaWO4�������ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С������ұ�������У����������Ƽ��������Ƽ�����Һ�еõ�����ƣ�������Ӧ��WO42-��aq��+Ca��OH��2��s���TCaWO4��s��+2OH-��aq������1��ͼΪ��ͬ�¶���Ca��OH��2��CaWO4�ij����ܽ�ƽ�����ߣ�
�ټ���T1ʱKSP��CaWO4��=1��10-10��
��T1�� T2���������=����������
��2����Ӧ���ƽ�ⳣ��K����ֵ�����
| �¶�/�� | 25 | 50 | 90 | 100 |
| K | 79.96 | 208.06 | 222.88 | 258.05 |
�ڸ÷�Ӧ�ġ�H��0���������=����������
��������Һ�����Ӽ������ã�ʵ���õ�ƽ�ⳣ��������ֵ�����Զ��50��ʱ����һ������������Ƽ�����Һ[c��Na2WO4��=c��NaOH��=0.5mol•L-1]�У��������Ca��OH��2����Ӧ�ﵽƽ���WO42-�ij�����Ϊ60%������ʵ���õ�ƽ�ⳣ����
��3����ȡ�����ʱ����ʱ��Ӧ���Һ�������������ᣬ���������ã��������ᣬ���ķ�Ӧ���ɵ�OH-��ʹ��Һ��OH-Ũ�ȼ�С��ƽ��������Ӧ�����ƶ������WO42-�ij����ʣ�
| A�� | �÷�Ӧ�����£�̼������������ˮ | B�� | �����Ͷ�����̼�����Ժϳɰ����� | ||
| C�� | �����Ƽʵ���˶���ѭ������ | D�� | ����ʱ��Ӧ��ͨ������̼��ͨ���� |
 ģ������Ƽԭ������CaCl2Ũ��Һ��ͨ��NH3��CO2���Ƶ��������ϣ�װ�ü�ͼʾ������˵����ȷ���ǣ�������
ģ������Ƽԭ������CaCl2Ũ��Һ��ͨ��NH3��CO2���Ƶ��������ϣ�װ�ü�ͼʾ������˵����ȷ���ǣ�������| A�� | aͨ��������CO2��bͨ��������NH3�����ײ���ΪCa��HCO3��2 | |
| B�� | aͨ��������NH3��bͨ��������CO2�����ײ���ΪCa��HCO3��2 | |
| C�� | aͨ��������CO2��bͨ��������NH3�����ײ���ΪCaCO3 | |
| D�� | aͨ��������NH3��bͨ��������CO2�����ײ���ΪCaCO3 |
| A�� | ���������ͬ�Ļ����� | B�� | ���ӹ�����ͬ�Ļ����� | ||
| C�� | ���������ͬ�����첻ͬ�Ļ����� | D�� | ��������빹�춼����ͬ�Ļ����� |
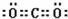 ��
��