��Ŀ����
10���л���X��һ����Ҫ���л��ϳ��м��壬�����������ϡ�Ϳ�Ϻ�ճ�ϼ��ȸ߾��Ϊ�о�X�������ṹ������������ʵ�飺��1���л���X ������ͼΪ�� | ��1���л���X����Է���������100�� |
| ��2����10.0g X������O2�г��ȼ�գ���ʹ���������ͨ����������ˮCaCl2��KOHŨ��Һ��������ˮCaCl2����7.2g��KOHŨ��Һ����22.0g�� | ��2���л���X�ķ���ʽ�� C5H8O2�� |
| ��3����������ײⶨ���л���X�к���ȩ�����л���X�ĺ˴Ź�������ͼ����2�����շ壬�����֮����3��1�� | ��3���л���X�Ľṹ��ʽ�� ��CH3��2C��CHO��2�� |
���� ��1������ͼ���ʺɱ������ֵ��Ϊ��Է���������
��2��10.0g X����Ԫ�ص����ʵ���=$\frac{7.2g}{18g/mol}$=0.4mol��KOHŨ��Һ����22.0g�Ƕ�����̼������������̼ԭ���غ�֪��̼Ԫ�ص����ʵ���=$\frac{22.0g}{44g/mol}$=0.4mol��ʣ�ಿ������Ԫ�أ�Ȼ������÷�����̼���⡢��Ԫ�صĸ���֮�ȣ�
��3���л���X�к���ȩ�����л���X�ĺ˴Ź�������ͼ����2�����շ壬������к���2�ֻ�������ԭ�ӣ��ݴ˷�����
��� �⣺��1������ͼ���ʺɱ������ֵ��Ϊ��Է���������������ͼ��֪�л���X����Է���������100���ʴ�Ϊ��100��
��2��10.0g X����Ԫ�ص����ʵ���=$\frac{7.2g}{18g/mol}$��2=0.8mol��KOHŨ��Һ����22.0g�Ƕ�����̼������������̼ԭ���غ�֪��̼Ԫ�ص����ʵ���=$\frac{22.0g}{44g/mol}$=0.5mol��ʣ�ಿ������Ԫ�أ���Ԫ�ص�����Ϊ10.0g-0.8��1g-0.5��12g=3.2g������Ԫ�ص����ʵ���Ϊ$\frac{3.2g}{16g/mol}$=0.2mol����÷�����̼���⡢��Ԫ�صĸ���֮��0.5mol��0.8mol��0.2mol=5��8��2�����л���X�ķ���ʽ��C5H8O2���ʴ�Ϊ��C5H8O2��
��3���л���X�к���ȩ�����л���X�ĺ˴Ź�������ͼ����2�����շ壬������к���2�ֻ�������ԭ�ӣ�����ṹ��ʽΪ��CH3��2C��CHO ��2��
�ʴ�Ϊ����CH3��2C��CHO ��2��
���� ���⿼���л���Ľṹ�����ʡ�����ʽ��ȷ��������ʽ����д�ȣ���Ŀ��Ϊ�ۺϣ���һ�����Ѷȣ�����ʱע��������йؼ���Ϣ���������������ϵķ����ƶϣ�ͬʱ����ѧ����������ͽ�������������

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�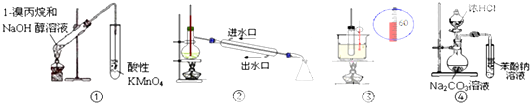
| A�� | ��װ�����ڼ���1-�������ȥ���� | B�� | ��װ������ʯ�͵ķ��� | ||
| C�� | ��װ�ÿ�֤�����ԣ����̼����� | D�� | ��װ������ʵ������������ |
| A�� | ��ϩ�ܷ����ӳɷ�Ӧ�����鲻�� | |
| B�� | ���ӣ�C6H5OH���ܸ�NaOH��Һ��Ӧ���Ҵ����� | |
| C�� | �ױ���ʹKMnO4������Һ��ɫ�����鲻�� | |
| D�� | ����50�桫60��ʱ����������Ӧ���ױ���30��ʱ���� |
| A�� | O��S��Se��Te | B�� | C��N��O��F | C�� | P��S��O��F | D�� | K��Na��Mg��Al |
| A�� | �����ĵ���ʽ  | |
| B�� | Mg2+�Ľṹʾ��ͼ  | |
| C�� | NH3�Ľṹʽ 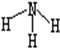 | |
| D�� | �õ���ʽ��ʾ�廯����γɹ���Ϊ��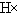 + +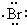 �� ��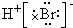 |
��1����֪����H2��g��+$\frac{1}{2}$O2��g���TH2O��l����H1�T-285.8KJ•mol-1
��CH3OH��l��+$\frac{3}{2}$O2��g���TCO2��g��+2H2O��l����H1�T-725.5KJ•mol-1
��CO2��g��+3H2��g���TCH2OH��l��+H2O��l���ķ�Ӧ�ȡ�H�T-131.9KJ/mol����
��2��ij�о�С����CO�ϳɼ״���CO��g��+2H2��g���TCH3OH��l����H��0��������������ֱ�ͨ�뵽����2L�����ܱ������н��з�Ӧ���õ���������ʵ�����ݣ�
| ʵ���� | �¶� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | �ﵽƽ�������ʱ��/min | ||||
| CO | H2 | CH3OH | CO | H2 | CH3OH | |||
| 1 | 650�� | 2.0 | 6.0 | 0 | 1.0 | 5 | ||
| 2 | 900�� | 2.0 | 6.0 | 0 | 1.2 | 2 | ||
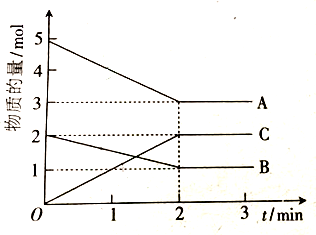
| 3 | 650�� | 1.0 | 4.0 | 2.0 | a | b | c | t |
��3����֪CO��ת���ʣ�a�����¶ȣ�T����ѹǿ��p���Ĺ�ϵ����ͼ��ʾ��
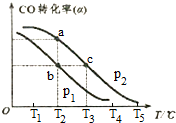
��p1��p2����������������T�������������¶ȣ�������ѹǿ���ܷ�ʵ��b�㵽c���ת�����ܣ���ܡ����ܡ�����ԭ�����������¶ȣ�������ѹǿ��һ����̼ת�����ȼ�С�������Ե���C��һ����̼ת���ʲ��䣮
��a��c����ķ�Ӧ����Ϊv1��v2����������������T����
���ڲ��ı䷴Ӧ������������£�Ϊ���CO��ת���ʿɲ�ȡ�Ĵ�ʩ�ǣ���������Ũ�ȣ�����ѹǿ�������¶ȣ����������ʩ��