��Ŀ����
7������ˮ����ζ����������A���Է������б仯��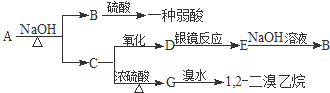
��1��д�����б仯�Ļ�ѧ����ʽ��
C��D��2C2H5OH+O2$��_{��}^{ͭ����}$2CH3CHO+2H2O
D��E��CH3CHO+2Ag��NH3��2OH$\stackrel{ˮԡ}{��}$CH3COONH4+2Ag��+3NH3+H2O
G�ļӾ۷�Ӧ��

ʵ������ȡA�ķ�Ӧ��

��2���л���A��ͬ���칹��������Na2CO3��Һ��Ӧ�ų�������̼�Ľṹ��ʽΪCH3CH2CH2COOH����CH3��2CHCOOH��
���� ����ˮ����ζ����������A�ܷ���ˮ�ⷴӦ����B��C��B�����ᷴӦ����һ�����ᣬ��B�������ƣ�C�Ǵ���C����������D��D�ܷ���������Ӧ����DΪȩ��D��������������E��E���������Ʒ�Ӧ����������B��������ʹ�������Cԭ����ȣ�C������ȥ��Ӧ����G��G���巢���ӳɷ�Ӧ����1��2-�������飬��G�ṹ��ʽΪCH2=CH2��C�ṹ��ʽΪCH3CH2OH��DΪCH3CHO��EΪCH3COOH��BΪCH3COONa��AΪCH3COOCH2CH3���ݴ˷������
��� �⣺����ˮ����ζ����������A�ܷ���ˮ�ⷴӦ����B��C��B�����ᷴӦ����һ�����ᣬ��B�������ƣ�C�Ǵ���C����������D��D�ܷ���������Ӧ����DΪȩ��D��������������E��E���������Ʒ�Ӧ����������B��������ʹ�������Cԭ����ȣ�C������ȥ��Ӧ����G��G���巢���ӳɷ�Ӧ����1��2-�������飬��G�ṹ��ʽΪCH2=CH2��C�ṹ��ʽΪCH3CH2OH��DΪCH3CHO��EΪCH3COOH��BΪCH3COONa��AΪCH3COOCH2CH3��
��1��C��D�Ļ�ѧ����ʽΪ2C2H5OH+O2$��_{��}^{ͭ����}$2CH3CHO+2H2O��D��E�Ļ�ѧ����ʽΪCH3CHO+2Ag��NH3��2OH$\stackrel{ˮԡ}{��}$CH3COONH4+2Ag��+3NH3+H2O��G�ļӾ۷�Ӧ�Ļ�ѧ����ʽΪ ��ʵ������ȡA�ķ�Ӧ�Ļ�ѧ����ʽΪ
��ʵ������ȡA�ķ�Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ��2C2H5OH+O2$��_{��}^{ͭ����}$2CH3CHO+2H2O��CH3CHO+2Ag��NH3��2OH$\stackrel{ˮԡ}{��}$CH3COONH4+2Ag��+3NH3+H2O�� ��
�� ��
��
��2��AΪCH3COOCH2CH3��A��ͬ���칹��������Na2CO3��Һ��Ӧ�ų�������̼����ýṹ�����Ȼ��������Ľṹ��ʽ��CH3CH2CH2COOH����CH3��2CHCOOH��
�ʴ�Ϊ��CH3CH2CH2COOH����CH3��2CHCOOH��
���� ���⿼�黯ѧ��Ӧ����ʽ����д����ȷ�����Ļ�ѧ��Ӧ�ǽ����Ĺؼ���ע�ⳣ���л�������ʣ��ѶȲ���

| A�� | 4 | B�� | 1 | C�� | 2 | D�� | 3 |
| A�� | ��ϩ��ʵ��ʽ��C2H4 | B�� | ��Ȳ�ĵ���ʽ��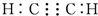 | ||
| C�� | �Ҵ��Ľṹ��ʽ��C2H6O | D�� | ����������ʽ��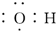 |
| A�� | N2��NH3������������� | B�� | N2��H2��NH3��Ũ��֮��Ϊ1��3��2 | ||
| C�� | ��Ӧ���������Ũ�Ȳ��ٷ����仯 | D�� | ����Ӧ���淴Ӧ���ٽ��� |

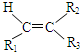 $��_{��Zn/H_{2}O}^{��O_{3}}$ R1CHO+
$��_{��Zn/H_{2}O}^{��O_{3}}$ R1CHO+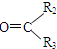
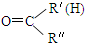 $\stackrel{һ��������}{��}$ R-
$\stackrel{һ��������}{��}$ R-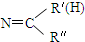 +H2O
+H2O ��
��

 ��
��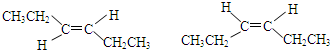 ��
��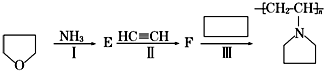
 ��F
��F

 ��
�� ���û������������ӣ�����ۡ������ӡ��������
���û������������ӣ�����ۡ������ӡ��������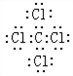 ���û��������ɼ��ԣ�����ԡ��Ǽ��ԡ������γɵģ�
���û��������ɼ��ԣ�����ԡ��Ǽ��ԡ������γɵģ�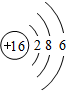 ��
��