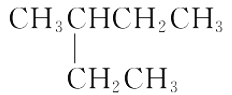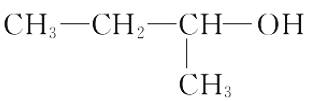��Ŀ����
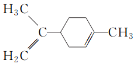
����Ŀ��A��B��C��D���ַ����廯������ǵĽṹ��ʽ������ʾ��
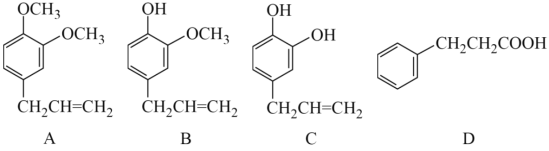
����д���пհף�
(1)A�ķ���ʽΪ______________�� D�к��������ŵ�������____________��
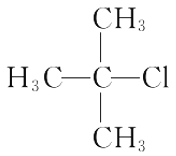
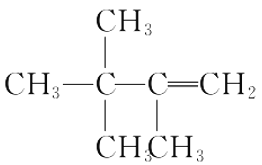
(2)�������ֻ������У���Ϊͬ���칹�����______������ĸ���ţ���
(3)�����ڼ���A��C����______������ţ���
�����Ը��������Һ ��̼��������Һ ���Ȼ�����Һ
(4)DΪԭ�Ϻϳ�ij�������ϵ��������£�
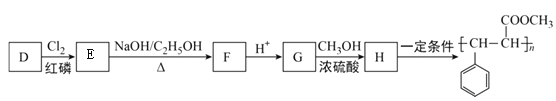
��ʾ��R-CH2-COOH+Cl2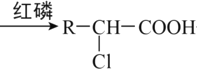 +HCl
+HCl
��D��E�ķ�Ӧ����_________________��
��F�Ľṹ��ʽ_________________��
��E��F��Ӧ�Ļ�ѧ����ʽ��________________��
��G��H��Ӧ�Ļ�ѧ����ʽ��_________________��
���𰸡�C11H14O2 �Ȼ� CD �� ȡ�� ![]()
![]() +2NaOH
+2NaOH![]()
![]() +NaCl+2H2O
+NaCl+2H2O ![]() +CH3OH
+CH3OH![]()
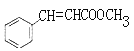 +H2O
+H2O
��������
(1)������ṹ��ʽȷ�������ʽ��D�к��й�����Ϊ�Ȼ���
(2)����ʽ��ͬ�ṹ��ͬ�����ʻ�Ϊͬ���칹�壻
(3)A�к����ѻ���C�к��з��ǻ������ݹ����ŵ���ͬ�������
(4)�����������������Ϣ�϶�ȡ����Ӧ����E��E�Ľṹ��ʽΪ![]() ��E������ȥ��Ӧ����F��F�Ľṹ��ʽΪ
��E������ȥ��Ӧ����F��F�Ľṹ��ʽΪ![]() ��F���ữ����G��G�Ľṹ��ʽΪ
��F���ữ����G��G�Ľṹ��ʽΪ![]() ��G�ͼ״�����������Ӧ����H��H�Ľṹ��ʽΪ
��G�ͼ״�����������Ӧ����H��H�Ľṹ��ʽΪ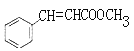 ��
��
(1)������ṹ��ʽ֪�����ʽΪC11H14O2��������ṹ��ʽ֪D�к��������������Ȼ���
(2)����ʽ��ͬ�ṹ��ͬ�����ʻ�Ϊͬ���칹�壬���ݽṹ��ʽ֪��C��D��Ϊͬ���칹�壻
(3)A�к����ѻ���̼̼˫����C�к��з��ǻ���̼̼˫����
�ٶ��߶�����̼̼˫�������Զ���ʹ���Ը��������Һ��ɫ�����������𣬢ٴ���
�ڶ��߶�����̼��������Һ��Ӧ�����������𣬢ڴ���
��A�к����ѻ���C�к��з��ǻ������ܺ��Ȼ�����Һ������ɫ��Ӧ���ѻ������Ȼ�����Һ������ɫ��Ӧ�����������Ȼ�����Һ���𣬢���ȷ��
�ʺ���ѡ���Ǣۣ�
(4)�����������������Ϣ�϶�ȡ����Ӧ����E��E�Ľṹ��ʽΪ![]() ��E������ȥ��Ӧ����F��F�Ľṹ��ʽΪ
��E������ȥ��Ӧ����F��F�Ľṹ��ʽΪ![]() ��F���ữ����G��G�Ľṹ��ʽΪ
��F���ữ����G��G�Ľṹ��ʽΪ![]() ��G�ͼ״�����������Ӧ����H��H�Ľṹ��ʽΪ
��G�ͼ״�����������Ӧ����H��H�Ľṹ��ʽΪ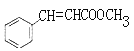 ��
��
��ͨ�����Ϸ���֪�����������������ȡ����Ӧ����E��![]() ���÷�Ӧ����ȡ����Ӧ��
���÷�Ӧ����ȡ����Ӧ��
��ͨ�����Ϸ���֪��F�Ľṹ��ʽΪ![]() ��
��
��E��![]() ��������NaOH���Ҵ���Һ���ȣ�������ȥ��Ӧ����F��E��F��Ӧ�Ļ�ѧ����ʽ��
��������NaOH���Ҵ���Һ���ȣ�������ȥ��Ӧ����F��E��F��Ӧ�Ļ�ѧ����ʽ��![]() +2NaOH
+2NaOH![]()
![]() +NaCl+2H2O��
+NaCl+2H2O��
��G�Ľṹ��ʽΪ![]() ��G�ͼ״�CH3OH��Ũ������ڲ�����ʱ����������Ӧ����H���÷�Ӧ����ʽΪ��
��G�ͼ״�CH3OH��Ũ������ڲ�����ʱ����������Ӧ����H���÷�Ӧ����ʽΪ��![]() +CH3OH
+CH3OH![]()
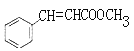 +H2O��
+H2O��

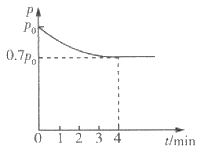
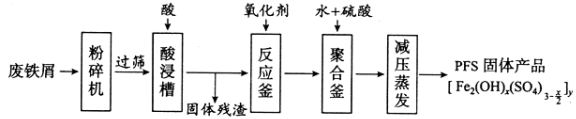
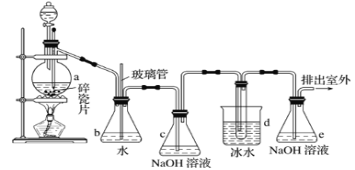
����Ŀ�������������Ӽ�����1��2-�������顣��ͼΪʵ�����Ʊ�1��2-���������װ�Dͼ�� ͼ�з�Һ©������ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ���dװ�D�Թ���װ��Һ�塣
��֪��CH3CH2OH![]() CH2=CH2��+H2O��
CH2=CH2��+H2O��
2CH3CH2OH![]() CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
��������б����£�
�Ҵ� | 1��2-�������� | ���� | �� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | ����ɫҺ�� |
�ܶ�/g��cm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
�е�/�� | 78.5 | 131.4 | 34.6 | 58.8 |
�۵�/�� | -114.3 | 9.79 | - 116.2 | -7.2 |
ˮ���� | ���� | ���� | �� | ���� |
(1)��ȫƿb��ʵ�����ж������á���һ���Լ��ʵ�������dװ�D�е����Ƿ�����������д����������ʱƿb�е�����_______________�����ʵ��ʱdװ�D�е��ܶ���������Ϊ���ܵ�ԭ���Ǣ�_______________����ȫƿb�������������Ǣ�_______________��
(2)����c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ��������_____________________��
(3)ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³������࣬���װ�D��������û�����⣬�Է������ܵ�ԭ��______________��______________(д����������)��
(4)��ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ___________��Ҫ��һ���ᴿ�����в����б������_____________ (����ĸ)��
A���ؽᾧ B������ C����ȡ D������
(5)ʵ����Ҳ���Գ�ȥdװ�D��ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�D���Թ��У����ʱ��ˮ����������ȴ1��2-��������������⣬��������������______��