��Ŀ����
��14�֣�������ϡ������п��ȡ������ʵ���У�������������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
��1��������������ͭ��Һ���Լӿ������������ʵ�ԭ���� ��
��2��Ҫ�ӿ�����ʵ����������������ʣ����ɲ�ȡ�Ĵ�ʩ�� �������֣���
��3��Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬ijѧϰС�����������һϵ��ʵ�顣�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䡣
����ɴ�ʵ����ƣ����У�V2 V5 �� �� V6�� ��V8�� ��
����һ����������������������������������ֵ��
�����£�ijһԪ��HA��NaOH��Һ�������ϣ�HA��NaOH��Ũ���Լ���Ϻ���Һ��pH���±���
��ش��������⣺
��4�����Ӽ������������c�Ƿ�һ������0.2 �� ��ѡ��ǡ�����
��5����������ʵ�����ݣ�HA�� �ᣨѡ�ǿ�������������û��Һ������Ũ���ɴ�С��˳���� ��
��6���������û��Һ����ˮ�������c(OH-) = mo1��L-1��
��1��������������ͭ��Һ���Լӿ������������ʵ�ԭ���� ��
��2��Ҫ�ӿ�����ʵ����������������ʣ����ɲ�ȡ�Ĵ�ʩ�� �������֣���
��3��Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬ijѧϰС�����������һϵ��ʵ�顣�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䡣
| ʵ�� �����Һ | A | B | C | D | E | F |
| 4 mol��L-1H2SO4 / mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ / mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O / mL | V7 | V8 | V9 | 15 | 10 | 0 |
����һ����������������������������������ֵ��
�����£�ijһԪ��HA��NaOH��Һ�������ϣ�HA��NaOH��Ũ���Լ���Ϻ���Һ��pH���±���
| ��� | c��HA��/mo1��L-1 | c��NaOH��/mo1��L-1 | ���ҺpH |
| �� | c | 0.2 | pH = 7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH = 9 |
��4�����Ӽ������������c�Ƿ�һ������0.2 �� ��ѡ��ǡ�����
��5����������ʵ�����ݣ�HA�� �ᣨѡ�ǿ�������������û��Һ������Ũ���ɴ�С��˳���� ��
��6���������û��Һ����ˮ�������c(OH-) = mo1��L-1��
��14�֣�
 ��4����1�֣���
��4����1�֣���
��5������1�֣���c(Na+)��c(A��)��c(OH��)��c(H+)���� [Na+]��[A��]��[OH��]��[H+]����2�֣�
��6��10-5 ��2�֣���
 ��4����1�֣���
��4����1�֣�����5������1�֣���c(Na+)��c(A��)��c(OH��)��c(H+)���� [Na+]��[A��]��[OH��]��[H+]����2�֣�
��6��10-5 ��2�֣���
��1��
��2���ӿ컯ѧ��Ӧ�����ʵ������У������¶ȡ��������������ѹǿ��������μӵķ�Ӧ��������Ӧ���Ũ�ȡ���Ӧ��ĽӴ������С�ȣ�����ɲ��õĴ�ʩ�У�
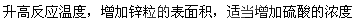
��3����ʵ���Ŀ��Ϊ���о�����ͭ�����������������ʵ�Ӱ�죬������ȡ�������������ͬ�ģ���V2=V5 ��30��
�����ӱ���CuSO4��Һ�����ݷ����ɵã�V6��10���Ƚ�D��E��F�б���CuSO4��Һ�����������ˮ�����������֪������CuSO4��Һ����������ˮ�������Ϊ20���������V8��19.5��
��4�����ڼ�����˵����Һ��pH = 7Ϊ������Һ������ȷ��c�Ƿ�һ������0.2��HA����Ϊ���ᣬҲ����Ϊǿ�
��5����������pH��7����Һ�ʼ��ԣ����ڵ������ϵ�����£�HA��Ũ������NaOH��Һ��2�������ɵ�HAһ��Ϊ���ᡣ�û����ϵ�У���Һ�е�����Ϊ�����ʵ�����HA��NaA��
��Һ�е����ӳɷ�Ϊ��Na+��A-��H+��OH-���ֳɷ֣�������Һ�ʼ��ԣ�����A-ˮ��Ϊ��HA����Ϊ�Σ�������Һ�и�����Ũ�ȵĴ�С��ϵΪ��c(Na+)��c(A��)��c(OH��)��c(H+)
��6������Ϊ����������ʵ���Ũ�ȵ�һԪ����һԪ���ϣ���Ϻ���Һ�е�����ΪNaA������Һ��pH = 9����HAΪ���ᣬA��ˮ��ʹ��Һ�ʼ��ԣ��ٽ�ˮ�ĵ��롣ˮ�������c(OH-) =10-5

��2���ӿ컯ѧ��Ӧ�����ʵ������У������¶ȡ��������������ѹǿ��������μӵķ�Ӧ��������Ӧ���Ũ�ȡ���Ӧ��ĽӴ������С�ȣ�����ɲ��õĴ�ʩ�У�
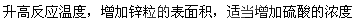
��3����ʵ���Ŀ��Ϊ���о�����ͭ�����������������ʵ�Ӱ�죬������ȡ�������������ͬ�ģ���V2=V5 ��30��
�����ӱ���CuSO4��Һ�����ݷ����ɵã�V6��10���Ƚ�D��E��F�б���CuSO4��Һ�����������ˮ�����������֪������CuSO4��Һ����������ˮ�������Ϊ20���������V8��19.5��
��4�����ڼ�����˵����Һ��pH = 7Ϊ������Һ������ȷ��c�Ƿ�һ������0.2��HA����Ϊ���ᣬҲ����Ϊǿ�
��5����������pH��7����Һ�ʼ��ԣ����ڵ������ϵ�����£�HA��Ũ������NaOH��Һ��2�������ɵ�HAһ��Ϊ���ᡣ�û����ϵ�У���Һ�е�����Ϊ�����ʵ�����HA��NaA��
��Һ�е����ӳɷ�Ϊ��Na+��A-��H+��OH-���ֳɷ֣�������Һ�ʼ��ԣ�����A-ˮ��Ϊ��HA����Ϊ�Σ�������Һ�и�����Ũ�ȵĴ�С��ϵΪ��c(Na+)��c(A��)��c(OH��)��c(H+)
��6������Ϊ����������ʵ���Ũ�ȵ�һԪ����һԪ���ϣ���Ϻ���Һ�е�����ΪNaA������Һ��pH = 9����HAΪ���ᣬA��ˮ��ʹ��Һ�ʼ��ԣ��ٽ�ˮ�ĵ��롣ˮ�������c(OH-) =10-5

��ϰ��ϵ�д�
�����Ŀ
 pC��g����qD��g����
pC��g����qD��g����
 2C���ڷ�Ӧ������C�����ʵ����������¶ȱ仯��ͼ��ʾ��
2C���ڷ�Ӧ������C�����ʵ����������¶ȱ仯��ͼ��ʾ��
 3C(g)�����¶�һ������A��g����B��g����ʼ��Ӧ������˵����ȷ����
3C(g)�����¶�һ������A��g����B��g����ʼ��Ӧ������˵����ȷ����

 ��H���ĵ���ƽ�ⳣ��K��____________������֪��10��5.6��2��5��10��6����
��H���ĵ���ƽ�ⳣ��K��____________������֪��10��5.6��2��5��10��6����
 ���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ���� ��
���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ���� �� �¶��£�0.1mol/LFeCl3��Һ��Fe3+ˮ��ٷ�����0.01mol��L��1FeCl3��Һ��Fe3+��ˮ��ٷ��ʣ� ��
�¶��£�0.1mol/LFeCl3��Һ��Fe3+ˮ��ٷ�����0.01mol��L��1FeCl3��Һ��Fe3+��ˮ��ٷ��ʣ� �� ԭ����Һ��ԭ����Һ�����ʵ���Ũ�ȣ� ��
ԭ����Һ��ԭ����Һ�����ʵ���Ũ�ȣ� ��