��Ŀ����
��6�֣�(1)��һ�������£�mA+nB=pC�ķ�Ӧ�У������ʵĻ�ѧ��Ӧ����ΪV(A)=amol��L-1��s-1,
V(B)=a/2mol��L-1��s-1, V(C)=amol��L-1��s-1,���ݻ�ѧ��Ӧ�����Ƴ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��10�֣�(2)��֪����ط����ֽⷴӦʱ���ͷų��������Ȼ��أ�����Ӧ���ʽϵ͡�
������Щ���������������صķֽⷴӦ���ʣ�
��д������طֽ�Ļ�ѧ��Ӧ����ʽ��
V(B)=a/2mol��L-1��s-1, V(C)=amol��L-1��s-1,���ݻ�ѧ��Ӧ�����Ƴ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��10�֣�(2)��֪����ط����ֽⷴӦʱ���ͷų��������Ȼ��أ�����Ӧ���ʽϵ͡�
������Щ���������������صķֽⷴӦ���ʣ�
��д������طֽ�Ļ�ѧ��Ӧ����ʽ��
(1)2A+B===2C (6��)
��2���������¶ȡ�ʹ�ô�������������гɷ�ĩ�ȣ�6�ִ���������ȫ��6�֣�
��2KClO3 =====2KCl + 3O2�� ��4�֣�
��2���������¶ȡ�ʹ�ô�������������гɷ�ĩ�ȣ�6�ִ���������ȫ��6�֣�
��2KClO3 =====2KCl + 3O2�� ��4�֣�
V(A):V(B):V(C)=2:1:2�ó�2A+B===2C�÷�Ӧ�Ǹ����ȷ�Ӧ�����������ӵķ�Ӧ�����Կ������¡�ʹ�ô���������Ӧ��ĽӴ��������������гɷ�ĩ��

��ϰ��ϵ�д�
�����Ŀ


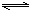 2NH3���䷴Ӧ�����ʿɷֱ��ʾΪv(H2)��v(N2)��v(NH3)(��λΪmol��L��1��s��1)�������й�ϵ��ȷ����
2NH3���䷴Ӧ�����ʿɷֱ��ʾΪv(H2)��v(N2)��v(NH3)(��λΪmol��L��1��s��1)�������й�ϵ��ȷ����