��Ŀ����
����Ŀ��A��B��C��D��E��FΪǰ������Ԫ����ԭ������������������A����3���ܼ�����ÿ���ܼ������ĵ�������ͬ��C���������6���˶�״̬��ͬ�ĵ��ӣ�D�Ƕ�����Ԫ���е縺����С��Ԫ�أ�E������������Ӧ��ˮ����������ǿ��F�������ԭ�ӹ�����ڰ����״̬�������ܲ���������ӡ�GԪ����DԪ��ͬ���壬�����3�����ڡ�
(1)�õ���ʽ��ʾC���⻯����γɹ���_________(��Ԫ�ط��ű�ʾ)��
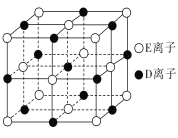
(2)Fԭ�ӵ���Χ�����Ų�ʽΪ________��F�ľ�����ԭ�ӵĶѻ���ʽ����ͼ�е�______����������������������������

(3)D��E��G��E�γɵľ���������ͬ�����������λ����ͬ����ԭ����________��
(4)��֪DE����ľ�����ͼ��ʾ������DE�����е�����E����ȥ��������D����ȫ����ΪAԭ�ӣ��������е�4����С�����������ĸ�����һ��Aԭ�ӣ�����4����С�������������ڡ�λ����С���������е�Aԭ���������4��Aԭ���Ե����������ɴ˱�ʾA��һ�־���ľ�������֪A-A���ļ���Ϊa cm��NA��ʾ�����ӵ�����������þ����к���________��Aԭ�ӣ��þ�����ܶ���________g/cm3��
���𰸡�![]() 3d104s1 �� ���������ӵİ뾶�Ȳ�ͬ 8
3d104s1 �� ���������ӵİ뾶�Ȳ�ͬ 8 ![]()
��������
A��B��C��D��E��FΪǰ������Ԫ����ԭ������������������A����3���ܼ�����ÿ���ܼ������ĵ�������ͬ������ԭ�Ӻ�������Ų�Ϊ1s22s22p2����AΪ̼Ԫ�أ�D�Ƕ�����Ԫ���е縺����С��Ԫ�أ���DΪNaԪ�أ�C���������6���˶�״̬��ͬ�ĵ��ӣ����ڢ�A�壬���ԭ��������ϵ��֪CΪ��Ԫ�أ���BΪNԪ�أ�E������������ˮ����������ǿ����EΪ��Ԫ�أ�F�������ԭ�ӹ�����ڰ����״̬�������ܲ���������ӣ�F�������ԭ�ӹ�����ڰ����״̬�������ܲ���������ӣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d104s1����FΪͭԪ�أ�GԪ����DԪ��ͬ���壬�����3�����ڣ���GΪ�Ԫ�أ��Դ˽����⡣
��������������֪A��CԪ�أ�B��NԪ�أ�C��OԪ�أ�D��NaԪ�أ�E��ClԪ�أ�F��CuԪ�أ�G��CsԪ�ء�
(1)C��OԪ�أ����⻯��H2O�ǹ��ۻ����H��Oԭ�Ӽ��Թ��ۼ���ϣ��õ���ʽ��ʾH2O���γɹ���Ϊ��![]() ��
��
(2)F��CuԪ�أ���������Ų�Ϊ1s22s22p63s23p63d104s1��ԭ�ӵ���Χ�����Ų�ʽΪ3d104s1��Cu�ľ�����ԭ�ӵĶѻ���ʽ�������������ܶѻ�������ͼ�еı���ʾ��
(3)D��Na��E��Cl��G��Cs��D��E�γɻ�������NaCl��G��E�γɵĻ������CsCl�����߶������Ӿ��壬�������������ӵİ뾶�Ȳ�ͬ�������ǵ���λ����ͬ��
(4)D��Na��E��Cl��D��E�γɻ�������NaCl�� NaCl����ľ����е�����Na+����ȥ��������Na+����ȫ����ΪC(̼)ԭ�ӣ��������е�4����С�����������ĸ�����һ��C(̼)ԭ�ӣ�����4����С�������������ڣ�λ����С���������е�Cԭ���������4��Cԭ���Ե����������γ���������ṹ���þ����к��е�C(̼)ԭ����Ŀ=4+8��![]() +6��
+6��![]() =8����������=8��
=8����������=8��![]() g��C-C���ļ���Ϊa cm����ͼ��������
g��C-C���ļ���Ϊa cm����ͼ��������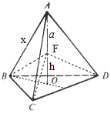 ������F��A��B��C��D��������A-BCD����Ϊ��4�����������������V����A-BCD=4V����F-BCD��V����F-BCD=
������F��A��B��C��D��������A-BCD������4�����������������V����A-BCD=4V����F-BCD��V����F-BCD=![]() S��BCD��(a+h)=4��
S��BCD��(a+h)=4��![]() S��BCD��h������h=
S��BCD��h������h=![]() ��������������ⳤΪx cm������AF���ӳ���������BCD��O��OΪ��������BCD�����ģ�BO��ֱƽ��CD����BO�ij���=xcm��
��������������ⳤΪx cm������AF���ӳ���������BCD��O��OΪ��������BCD�����ģ�BO��ֱƽ��CD����BO�ij���=xcm��![]() ��
��![]() =
=![]() cm����ֱ��������BOF�У�(
cm����ֱ��������BOF�У�(![]() )2+(
)2+(![]() )2=a2�����x=
)2=a2�����x=![]() ���ʾ������ⳤ=
���ʾ������ⳤ=![]() ��2x cm=
��2x cm=![]() ��2��
��2��![]() cm=
cm=![]() cm���ʾ��������=(
cm���ʾ��������=(![]() cm)3=
cm)3=![]() a3 cm3�����ܶ�=
a3 cm3�����ܶ�=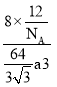 g/cm3=
g/cm3=![]() g/cm3��
g/cm3��

����Ŀ��![]() �ڹ�ҵ��ũҵ�ȷ����й㷺��Ӧ�ã���ҵ�Ͽ��ɸ������̿���Ҫ�ɷ�Ϊ
�ڹ�ҵ��ũҵ�ȷ����й㷺��Ӧ�ã���ҵ�Ͽ��ɸ������̿���Ҫ�ɷ�Ϊ![]() ������
������![]() ��
��![]() ��
��![]() ��
��![]() �����ʣ��Ʊ������ֹ���������ͼ��ʾ��
�����ʣ��Ʊ������ֹ���������ͼ��ʾ��

��ؽ������������������������![]() ����ʼ������
����ʼ������![]() ������Ũ��Ϊ
������Ũ��Ϊ![]() ���㣩��
���㣩��
�������� |
|
|
|
|
|
��ʼ������ | 8.1 | 6.3 | 1.5 | 3.4 | 8.9 |
������ȫ�� | 10.1 | 8.3 | 2.8 | 4.7 | 10.9 |
��1����������ʱ������Ӧ�����ӷ���ʽΪ______________��
��2������![]() ����Χ��5��6���õ�����2����Ҫ�ɷֳ�
����Χ��5��6���õ�����2����Ҫ�ɷֳ�![]() ���_____________��
���_____________��
��3�������ӡ������м���![]() ��Ŀ����______��
��Ŀ����______��
��4�������̡������з�����Ӧ�Ļ�ѧ����ʽΪ______��
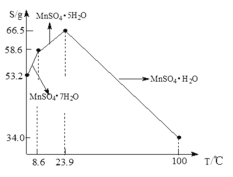
��5��![]() ��ˮ�е��ܽ�����¶ȹ�ϵ��ͼ����
��ˮ�е��ܽ�����¶ȹ�ϵ��ͼ����![]() ��ýϴ�����
��ýϴ�����![]() ����ķ����ǣ���
����ķ����ǣ���![]() ����������ϡ���ᣬ�����¶���
����������ϡ���ᣬ�����¶���![]() ֮�������ᾧ��______���õ�
֮�������ᾧ��______���õ�![]() ���壬ϴ�ӡ���ɡ�����ͨ�����ü�ѹ��ɵ�ԭ����______��
���壬ϴ�ӡ���ɡ�����ͨ�����ü�ѹ��ɵ�ԭ����______��
����Ŀ����0.1mol/LNa2SO3��Һ�������ٽ��£��ⶨ�¶ȱ仯�����е�pH���������£�
ʱ�� | �� | �� | �� | �� |
�¶�/�� | 25 | 30 | 40 | 25 |
pH | 9.66 | 9.52 | 9.37 | 9.25 |
��1����ʱ��Na2SO3��Һ��ˮ�ĵ���̶�________ͬ���´�ˮ��ˮ�ĵ���̶ȣ�����>������<������=������Ӧ��ƽ��ԭ������ԭ��_______________________________��
��2���ܵ�pH��С�ڢ�������_______________________________��