��Ŀ����
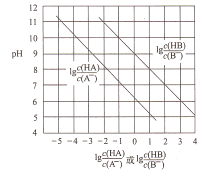
����Ŀ��Hg ��ˮ����Ⱦ���ؽ���Ԫ��֮һ��ˮ��Һ�еĶ��۹�����Ҫ������̬��Cl-��OH-��Ũ�ȹ�ϵ��ͼ��ʾ[ͼ���漰�����ʻ�����ֻ��Hg(OH)2 Ϊ���������Ũ�Ⱥ�Сʱ���ø�������ʾ����pH=-lgc(H+)��pCl=-1gc(Cl-)]:

����˵���д������
A. Hg(NO3)2��������ˮͨ������ֻ���
B. ��ˮCl-��Ũ�ȴ���0.1mol/L�������й�Ԫ�ص���Ҫ������̬��Hg(OH)2
C. ����Hg(NO3)2����0.001moL/L�����õ���������Һ
D. ��֪Ksp(HgS)=1.6��10-52����c(S2-)=1��10-5mo/L ʱ��c(Hg2+)=1.6��10-47mo/L
���𰸡�B
��������Hg2+��pH��3��14ʱ����Hg(OH)2����ʽ���ڣ���Hg(NO3)2��������ˮ��pH=7��ͨ������ֻ��ǣ���A����ˮCl-��Ũ�ȵ���0.1mol/Lʱ��pCl=-1gc(Cl-)=1����Cl-��Ũ�ȴ���0.1mol/L��pClΪ��С����ͼ��֪��Hg2+��HgCl42��������ʽ���ڣ���B����0.001moL/L������pH=3��pCl=3����ͼ��֪��Hg2+��Ҫ������ʽΪHgCl2����ҺΪ����������C��ȷ��Ksp(HgS)= c(S2-)c(Hg2+)=1.6��10-52����c(S2-)=1��10-5mo/Lʱ��c(Hg2+)= Ksp(HgS)/ c(S2-)=1.6��10-52/1��10-5mo/L=1.6��10-47mo/L����D��ȷ��

 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�����Ŀ��25��ʱ����������ĵ���ƽ�ⳣ�����£�
���� | CH3COOH | H2CO3 | H2SO3 |
����ƽ�ⳣ�� | K=1.8��10-5 | K1=4.3��10-7 K2=5.6��10-11 | K1=1.5��10-2 K2=1.02��10-7 |
��1��CH3COOH��H2CO3��H2SO3��������������������__________(�ѧʽ)��
��2��CH3COOH�ĵ���ƽ�ⳣ������ʽΪK=__________��
��3��д��H2CO3�ĵڶ������뷽��ʽ��_____________��
��4��д��H2SO3��Һ��CH3COONa��Һ��Ӧ�����ӷ���ʽ��____________________��
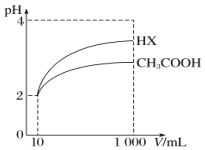
��5���������Ϊ10mL��pH��Ϊ2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1000mL��ϡ������pH�ı仯��ͼ��ʾ����HX�ĵ���ƽ�ⳣ��____________(����ڡ���С�ڡ����ڡ�)����ĵ���ƽ�ⳣ����������__________________��
����Ŀ����ȥ���������ڵ����ʣ������Լ��Ͳ�������ȷ����
ѡ�� | ����ӵ����� | �Լ� | ���� |
A | Cl2��HCl�� | ����ʳ��ˮ | ϴ�� |
B | NaBr��Һ��NaI�� | Cl2 | ϴ�� |
C | Cl2��H2O�� | ��ʯ�� | ϴ�� |
D | HNO3��Һ��H2SO4�� | BaCl 2��Һ | ���� |
A.AB.BC.CD.D