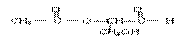��Ŀ����
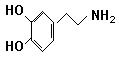
��A���������ʽṹ�����ʡ�
�±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
�Իش��������⣺
��1����д��Ԫ��D�Ļ�̬ԭ�ӵ����Ų�ʽ ��
��2��D��E��Ԫ�صIJ��ֵ��������������±���
�Ƚ���Ԫ�ص�I2��I3��֪����̬D2����ʧȥһ�����ӱ���̬E2����ʧȥһ�������ѡ��Դˣ���Ľ����� ��
��3��A���⻯�������ԭ�ӵ��ӻ���ʽΪ ��C�ڿ�����ȼ�ղ���ķ��ӹ���Ϊ �����以Ϊ�ȵ�����ĵ��ʵķ���ʽΪ ��
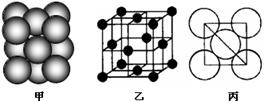
��4��B���ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��������Bԭ�ӵ���λ��Ϊ ��һ��������Bԭ�ӵ���ĿΪ ��
��B������ʵ�黯ѧ��
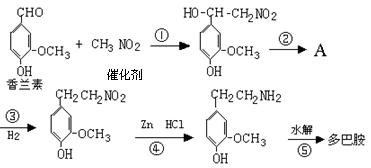
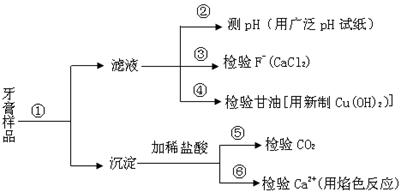
�������������Ʒ��������������ijЩ��Ҫ�ɷֵļ�������ͼ����
��ش��������⣺
��1���ڢ��м�ˮ�����衢���ú�������ʵ����������� ������Ҫ����Ҫ���������� ��
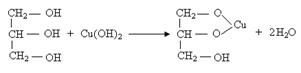
��2���ڢ�����pH��ֽ����Һ��pH�IJ��������� ��
��3��д�����з�����Ӧ�Ļ�ѧ����
ʽ�� ��
��4�������зų�������ͨ������ʯ��ˮ��ʱ��δ���������֣�����ܵ�ԭ���� ��
�����������
��5���ڢ��м���Ca2+�Ĵ��ڣ���������ɫ��Ӧ�⣬��������Ca2+�� ��Һ�ķ�Ӧ�����С�

�±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
| | | | |||||||||||||||
| | | | | | A | | | | |||||||||
| | | B | | | C | | | ||||||||||
| | | | | | | D | E | | | | | | | | | | |
��1����д��Ԫ��D�Ļ�̬ԭ�ӵ����Ų�ʽ ��
��2��D��E��Ԫ�صIJ��ֵ��������������±���
| Ԫ �� | D | E | |
| ������ /kJ��mol��1 | I1 | 717 | 759 |
| I2 | 1509 | 1561 | |
| I3 | 3248 | 2957 | |
��3��A���⻯�������ԭ�ӵ��ӻ���ʽΪ ��C�ڿ�����ȼ�ղ���ķ��ӹ���Ϊ �����以Ϊ�ȵ�����ĵ��ʵķ���ʽΪ ��
��4��B���ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��������Bԭ�ӵ���λ��Ϊ ��һ��������Bԭ�ӵ���ĿΪ ��
��B������ʵ�黯ѧ��
�������������Ʒ��������������ijЩ��Ҫ�ɷֵļ�������ͼ����

��ش��������⣺
��1���ڢ��м�ˮ�����衢���ú�������ʵ����������� ������Ҫ����Ҫ���������� ��
��2���ڢ�����pH��ֽ����Һ��pH�IJ��������� ��
��3��д�����з�����Ӧ�Ļ�ѧ����
ʽ�� ��
��4�������зų�������ͨ������ʯ��ˮ��ʱ��δ���������֣�����ܵ�ԭ���� ��
�����������
��5���ڢ��м���Ca2+�Ĵ��ڣ���������ɫ��Ӧ�⣬��������Ca2+�� ��Һ�ķ�Ӧ�����С�
��A����1��1s22s22p63s23p63d54s2 (2��)
��2�� Mn2+��3d��������Ų�Ϊ����״̬���ȶ� (2��)
��3��sp3 (2��) V�ͣ�����ͣ�(2��) O3 (2��)
��4�� 12 (1��) 4 (1��)
��B������1������������ ���������ձ� (4��)
��2���ò�����պȡ��������Һ������ڽྻ����������е�pH��ֽ�ϣ��������ɫ�����жԱ�(2��)
��3��
(2��)
��4�����ԣ�(2��)
��5������泥���GBHA���ԣ�(2��)

��2�� Mn2+��3d��������Ų�Ϊ����״̬���ȶ� (2��)
��3��sp3 (2��) V�ͣ�����ͣ�(2��) O3 (2��)
��4�� 12 (1��) 4 (1��)
��B������1������������ ���������ձ� (4��)
��2���ò�����պȡ��������Һ������ڽྻ����������е�pH��ֽ�ϣ��������ɫ�����жԱ�(2��)
��3��
(2��)
��4�����ԣ�(2��)
��5������泥���GBHA���ԣ�(2��)
��A��Ԫ��D��Mn��E��Fe��A���⻯����NH3��C�ڿ�����ȼ�ղ�����SO2��B��Al, ���ʾ�����ԭ�ӵĶѻ�Ϊͭ��

��B������1������������ ���������ձ� (4��)
��2���ò�����պȡ��������Һ������ڽྻ����������е�pH��ֽ�ϣ��������ɫ�����жԱ�(2��)
��3��
(2��)
��4�����ԣ�(2��)
��5������泥���GBHA���ԣ�(2��)

��B������1������������ ���������ձ� (4��)
��2���ò�����պȡ��������Һ������ڽྻ����������е�pH��ֽ�ϣ��������ɫ�����жԱ�(2��)
��3��
(2��)
��4�����ԣ�(2��)
��5������泥���GBHA���ԣ�(2��)

��ϰ��ϵ�д�
�����Ŀ