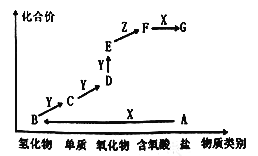
��Ŀ����
����Ŀ��������
(1)ij�ز�ʯ��ʯ�������ʲ�����ˮҲ�������ᷴӦ��Ϊ�ⶨ��ʯ��ʯ��̼��Ƶĺ������ֽ�������ʵ�飺��ȡ25gʯ��ʯ��������Ϊ145g���ձ��У������м���100gϡ���ᣬ��Ӧǡ����ȫ����ʱ�����ձ���������Һ��������Ϊ261.2g����
��ʯ��ʯ��̼��Ƶ�����Ϊ__________�ˡ�
�ڷ�Ӧ����Һ�����ʵ���������Ϊ__________(������λ��Ч����)��
(2)ij����ij������ĩ28.36 g������ֻ��Fe��C�������������г�ַ�Ӧ��������������ͨ�������ij���ʯ��ˮ�У��õ�3 g��ɫ������
�ټ���ɵô˸�����ĩ������̼������֮��Ϊ__________��
����ȡ���ݲ�ͬ�����ĸ�����ĩ�ֱ�ӵ�50g����������ͬ�������У���ַ�Ӧ��õ�ʵ���������±���ʾ������״���£�2�� H2�����Ϊ22.4L��
ʵ����� | �� | �� | �� |
���������ĩ������ / g | 2.836 | 5.672 | 8.508 |
����������������״����/ L | 1.12 | 2.24 | 2.80 |
���ݱ��е����ݼ��㣬������Һ��H2SO4���ʵ�����������__________
���𰸡�20 20%��0.20 700��9 24.5%��0.245
��������
(1)���������غ㶨�ɣ��ձ����������ʼ��ٵ������������ɵĶ�����̼���������ɶ�����̼������������̼��������ᷴӦ�ķ���ʽ�����̼��ơ��Ȼ��Ƶ����������������ݾͿ��Խ����йصļ��㡣
(2)̼����������Ӧ���ɶ�����̼��������̼��ʹ�����ʯ��ˮ����ǣ����������ᷴӦ�������������ݱ����ṩ�����ݿ�֪��ÿ2.836g������ĩ����������1.12L�������μ���8.508g����ֻ��������2.80L�����ʱ����ȫ���μӷ�Ӧ��������ʣ�࣬���Կ�֪50g���Ṳ��������2.80L����ʱ���Ը����������������������������������m������ĩֻ�����μӷ�Ӧ����òμӷ�Ӧ�������������������ʣ������������
���������֪����Ӧ�����ɵĶ�����̼������Ϊ��25g+145g+100g+100g-261.2g=8.8g�����ݷ�Ӧ����ʽCaCO3+2HCl=CaCl2+H2O+CO2����֪��ÿ100gCaCO3��Ӧ����44gCO2����Ӧ����8.8gCO2���壬��Ӧ����20gCaCO3��
�ڸ��ݷ�Ӧ����ʽCaCO3+2HCl=CaCl2+H2O+CO2����֪��ÿ100gCaCO3��Ӧ����111gCaCl2������44gCO2����Ӧ����8.8gCO2���壬��Ӧ��ͬʱ����22.2g CaCl2����Ӧ�������CaCl2����������Ϊ��![]() ��100%=20.0%��
��100%=20.0%��
(2)�� C+O2![]() CO2 ��
CO2 ��
CO2+Ca(OH)2=CaCO3��+H2O ��
���ݷ���ʽ�٢ڿɵù�ϵʽ C�� �� ��CaCO3��ÿ��Ӧ����100gCaCO3�跴Ӧ����12gC�������3g̼��Ƴ�����Ҫ̼������Ϊ![]() ��12g=0.36g�����28.36g�����к�����������Ϊ28.36g-0.36g=28g�����Ը�����ĩ������̼������֮��Ϊ��28g��0.36g=700��9��
��12g=0.36g�����28.36g�����к�����������Ϊ28.36g-0.36g=28g�����Ը�����ĩ������̼������֮��Ϊ��28g��0.36g=700��9��
�ڸ��ݱ����ṩ�����ݿ�֪��ÿ2.836g������ĩ����������1.12L�������μ���8.508g����ֻ��������2.80L��˵����ʱ����ȫ���μӷ�Ӧ��������ʣ�࣬���Կ�֪50g���Ṳ��������2.80L������������m(H2)=![]() ��2g=0.25g������Fe+H2SO4=FeSO4+H2�������ݷ���ʽ��֪��Ӧ����98g���ᣬ��Ӧ����2gH2����Ӧ����0.25gH2���������������m(H2SO4)=
��2g=0.25g������Fe+H2SO4=FeSO4+H2�������ݷ���ʽ��֪��Ӧ����98g���ᣬ��Ӧ����2gH2����Ӧ����0.25gH2���������������m(H2SO4)=![]() ��98g=12.25g�������������������Ϊ
��98g=12.25g�������������������Ϊ![]() ��100%=24.5%��
��100%=24.5%��

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д� Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�����Ŀ�������ʾΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã���ش��������⣺
��A | 0 | |||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | ||||
(1)�ۡ��ܡ��ߵ�ԭ�Ӱ뾶�ɴ�С��˳����________(��Ԫ�ط��ű�ʾ)��
(2)������ʵ��˵��Ԫ�آڵķǽ����Ա�Ԫ�آķǽ�����ǿ����________(����ĸ)��
A.�ڵĵ�����Ԫ�آļ��⻯����Һ��Ӧ����Һ�����
B.��������ԭ��Ӧ�У�1mol�ڵ��ʱ�1mol���ʵõ��Ӷ�
C.�ں͢���Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸ�
(3)�١�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ1��1��ɵij���Һ̬�������������Һ���ܽ�Fe2��������д���÷�Ӧ�����ӷ���ʽ�� ____��
(4)��֪���ڱ��д��ڶԽ����ƹ�������(Be)������ѧ�������ƣ�����������������Ҳ�����ԣ�д���������������ܵ�����������ˮ���ﷴӦ�Ļ�ѧ����ʽ��________��
(5)��֪W+X=Y+Z(��Ӧ��Ҫ����)��W��X��Y��Z�ֱ����ɢ٢ڢ�����Ԫ���γɵ�����10��������(W��XΪ���ӣ�Y��ZΪ����)��д�������ӷ���ʽ___________