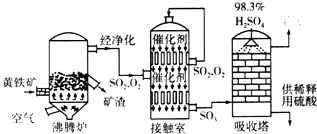
��Ŀ����
3���л���A���������̳��õ�ʳ�����ϣ�����Ϊԭ���Ʊ��߷���M����C4H6O��n�� N��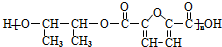 �ĺϳ�·����ͼ��ʾ��
�ĺϳ�·����ͼ��ʾ��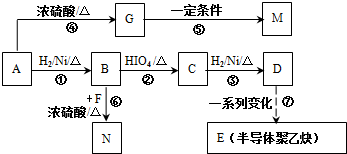
��֪������1mol A���ȼ�գ����������������A����160g��
����RCH=CHOH���ȶ�����ת��ΪRCH2CHO
����
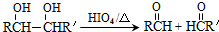
�밴Ҫ��ش��������⣺
��1����Ӧ�ݵķ�Ӧ���ͣ��Ӿ۷�Ӧ��B�ķ���ʽ��C4H10O2��
A�������������ƣ��ǻ����ʻ���A�����к��Ȼ���ͬ���칹��Ľṹ��ʽ����CH3��2CHCOOH��CH3CH2CH2COOH��M�Ľṹ��ʽ��
 ��
����2����Ӧ�Ļ�ѧ����ʽ��
 ��
����3��X�DZ�C��һ��CH2ԭ���ŵ�ͬϵ�д��X����������Ӧ�Ļ�ѧ����ʽ��HCHO+2Ag��NH3��2OH$\stackrel{��}{��}$ HCOONH4+2Ag��+3NH3+H2O��
��4�����Ƣ���������һϵ�б仯����������дԭ�ϡ�Ŀ�������м����Ľṹ��ʽ����ͷ�ϻ�����д��Ӧ�����Լ��ͷ�Ӧ��������

���� 1mol A���ȼ�գ����������������A����160g��������10molO�����M�ķ���ʽ��֪����A�ķ���ʽΪC4H8O2��A�в����Ͷ�=$\frac{4��2+2-8}{2}$=1�����Ժ���һ��˫����A�ܺ����������ӳɷ�Ӧ����B��B�ܺ�HIO4������Ӧ����������Ϣ��֪��B�к��д��ǻ���C�����������ӳɷ�Ӧ����D��CΪȩ����DΪ�Ҵ��ṹ��ʽΪCH3CH2OH��CΪCH3CHO��BΪCH3CH��OH��CH��OH��CH3��AΪCH3COCH��OH��CH3��B��F����������Ӧ����N��F�ṹ��ʽΪ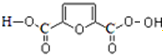 ��A������ȥ��Ӧ����G��G�ṹ��ʽΪCH3COCH=CH2��G�����Ӿ۷�Ӧ����M��M�ṹ��ʽΪ
��A������ȥ��Ӧ����G��G�ṹ��ʽΪCH3COCH=CH2��G�����Ӿ۷�Ӧ����M��M�ṹ��ʽΪ ���ݴ˷������
���ݴ˷������
��� �⣺1mol A���ȼ�գ����������������A����160g��������10molO�����M�ķ���ʽ��֪����A�ķ���ʽΪC4H8O2��A�в����Ͷ�=$\frac{4��2+2-8}{2}$=1�����Ժ���һ��˫����A�ܺ����������ӳɷ�Ӧ����B��B�ܺ�HIO4������Ӧ����������Ϣ��֪��B�к��д��ǻ���C�����������ӳɷ�Ӧ����D��CΪȩ����DΪ�Ҵ��ṹ��ʽΪCH3CH2OH��CΪCH3CHO��BΪCH3CH��OH��CH��OH��CH3��AΪCH3COCH��OH��CH3��B��F����������Ӧ����N��F�ṹ��ʽΪ�� ��A������ȥ��Ӧ����G��G�ṹ��ʽΪCH3COCH=CH2��G�����Ӿ۷�Ӧ����M��M�ṹ��ʽΪ
��A������ȥ��Ӧ����G��G�ṹ��ʽΪCH3COCH=CH2��G�����Ӿ۷�Ӧ����M��M�ṹ��ʽΪ ��
��
��1��ͨ�����Ϸ���֪����Ӧ�ݵķ�ӦΪ�Ӿ۷�Ӧ��B�ķ���ʽC4H10O2��A�к����ǻ����ʻ���A�����к��Ȼ���ͬ���칹��Ľṹ��ʽ��������2�֣�������2�֣���CH3��2CHCOOH��CH3CH2CH2COOH��M�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ���Ӿ۷�Ӧ�� C4H10O2���ǻ����ʻ�����CH3��2CHCOOH��CH3CH2CH2COOH�� ��
��
��2��BΪCH3CH��OH��CH��OH��CH3��B��F����������Ӧ����N����ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��3��CΪCH3CHO��X�DZ�C��һ��CH2ԭ���ŵ�ͬϵ���XΪHCHO����Ӧ����ʽΪ��HCHO+2Ag��NH3��2OH$\stackrel{��}{��}$ HCOONH4+2Ag��+3NH3+H2O��
�ʴ�Ϊ��HCHO+2Ag��NH3��2OH$\stackrel{��}{��}$ HCOONH4+2Ag��+3NH3+H2O��
��4��D��CH3CH2OH���м����1��Ũ����/170��CH2=CH2���м����2��������Ȼ�̼��Һ��CH2BrCH2Br���м����3��NaOH/�Ҵ�/���� CH��CH��E�� ��
��
�ʴ�Ϊ��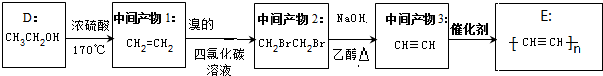 ��
��
���� ���⿼���л����ƶϣ�Ϊ�߿���Ƶ�㣬���ؿ���ѧ���������ƶ����������ݷ�Ӧ�����������Ϣ������ͼ�в������ʽṹʽ���������ϵķ��������ƶϣ��ѵ���ͬ���칹��������жϣ�Ҫ����̼���칹��������λ�ýṹ�ȣ�

| A�� | ԭ�Ӱ뾶��Y��Z��R��T | |
| B�� | ��̬�⻯����ȶ��ԣ�W��R��T | |
| C�� | ����������Ӧ��ˮ����ļ��ԣ�X��Z | |
| D�� | XR2��WR2���ֻ�������R�Ļ��ϼ���ͬ |
| A�� | ��CH3COONa��Һ�У��μӷ�̪��죺CH3COO-+H2O�TCH3COOH+OH- | |
| B�� | ��H2O2��Һ�У��μ�FeCl3��Һ�������ݣ�2H2O2+2Cl-�T2H2O+O2��+Cl2�� | |
| C�� | ����Ӵ���ͭƬ��пƬ����ϡ�����У�ͭƬ���������ݲ�����Cu+2H+�TCu2++H2�� | |
| D�� | ��Cu��OH��2����Һ�еμ�Na2S��Һ����ɫ�������ɫ��Cu��OH��2��s��+S2- ��aq��?CuS��s��+2OH- ��aq�� |
| A�� | �ں�����Fe3+����Һ�У�NH4+��Na+��Cl-��SCN- | |
| B�� | ������Һ�У�Na+��Cu2+��SO42-��NO3- | |
| C�� | ��c��H+��=10-13 mol/L����Һ�У�NH4+��Al3+��SO42-��CO32- | |
| D�� | ��pH=1����Һ�У�K+��Fe2+��Cl-��NO3- |
| ѡ�� | ����������ʵ | ������������ |
| A | ��������ȼ�����Ѭ������ | ���������л�ԭ�� |
| B | ����Ũ��ˮ��������ú���Ĺܵ��Ƿ���й© | ���ְ��̴���ú��й©�� |
| C | ������ĭ�����ʹ�õ���������������̼���� | ��������Ϸ���˫ˮ�ⷴӦ���ɶ�����̼���������� |
| D | ����ҽԺ�ﳣ���������ߵƽ���ɱ������ | ����������ʹ�����ʱ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 1 mol Cl2ͨ�뺬��1 mol FeBr2����Һ�У�2Fe2++2Br-+2Cl2�T2Fe3++4Cl-+Br2 | |
| B�� | ������ͭ�����缫������2H-+2Cl-$\frac{\underline{\;���\;}}{\;}$H2��+Cl2�� | |
| C�� | NH4HCO3��Һ�����NaOH��Һ��Ӧ��NH4++OH-�TNH3•H2O | |
| D�� | ��������Һ�м������������[Ag��NH3��2]++OH-+3H+�TAg++2NH4++H2O |
| A�� | 1molCnH2n+2�к��е�C-C����Ϊ��n-1��NA | |
| B�� | 4.2 g C3H6�к��е�̼̼˫����һ��Ϊ0.1 NA | |
| C�� | 1 mol-OH�е�����Ϊ10 NA | |
| D�� | ��״���£�2.24 L CHCl3��ԭ������Ϊ0.5 NA |