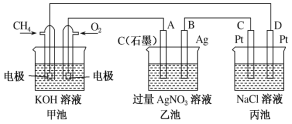
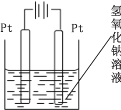
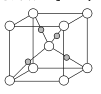
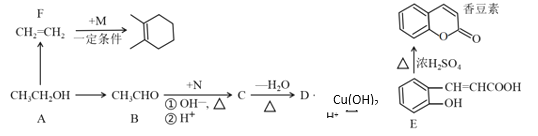
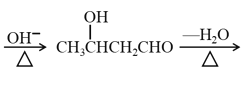��Ŀ����
����Ŀ������Fe3O4������ҽѧ�ʹ������������Ӧ��ǰ�����������Ʊ��������£�
��֪��п��������ǿ������ZnO22-��Zn��OH��2��������ǿ����������ǿ�
��ش��������⣺
(l)��NaOH��Һ�����Ͼ�п��Ƥ��������___��
A.ȥ������ B���ܽ��п�� C��ȥ������ D���ۻ�
(2)���������Zn(OH)2���������ӷ���ʽΪ____�������ӷ���ʽ�������˵���ò���pH���ܹ�С��ԭ��____������pH����ѷ���������Һ��ͨ��____���ѧʽ����
(3)����ܷ�Ӧ�����ӷ���ʽΪ_____��Ϊȷ������Fe3O4���ӵĴ��ȣ�Ӧ����ԭ��Һ��Fe2+������H2O2�����ʵ���֮��Ϊ_______��
(4)������Ƶ�Fe3O4�������ӵĹ��̣���Ҫ�����������½��У�˵������__________��Tҵ�����пɲ�ȡ___________��ʩ�ṩ����������
(5)����� _______����ܡ����ܡ����ü�ѹ���ˣ����ˣ��õ�����Fe3O4���ӣ�������___________��
���𰸡�AB ZnO22-+2H+=Zn(OH)2�� ��������Ϊ��ʹZnO22-ת��ΪZn��OH��2��������������ܹ��࣬Ҫ��ֹ����Zn��OH��2+2H+��Zn2++2H2O������ZnO���� CO2 2Fe2++H2O2+2H+=2Fe3++2H2O 3:1 ��ֹFe2+[��Fe��OH��2]������ ����ͨ��N ���� ��������̫С������ʱ��������ֽ
��������
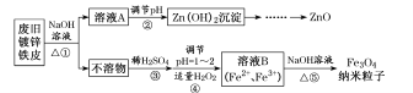
��������ͼ����֪��Ϣ�����ã��Ͼɶ�п��Ƥ��������������Һ�з�Ӧ��п�ܽ�����ƫп���ƺ������������ܽ⣬���˵õ���ҺAΪNa2ZnO2��������ΪFe����ҺA��ϡ����ʹ��Һ��ZnO22-ת��ΪZn��OH��2�������پ������ˡ�ϴ�ӡ�������յõ�ZnO��������Fe�м���ϡ���ᣬ��Ӧ�����Ȼ���������������H2O2������������������Ϊ�����ӣ��õ���Fe2+��Fe3+��B��Һ���ټ���NaOH����ͨ�뵪���ų����������ȷֽ⣬���������������������ӣ��ݴ˷������
��1������������Һ��������Ƥ���������ˮ���ȥ��ͬʱ�������Ϣ֪п����������������Һ���ʴ�Ϊ��AB��
��2�����������Ϣ֪�������ΪZnO22-���ᷴӦ����Zn��OH��2�ķ�Ӧ�����ӷ���ʽΪ��ZnO22-+2H+=Zn(OH)2������������Ϊ��ʹZnO22-ת��ΪZn��OH��2��������������ܹ��࣬Ҫ��ֹ����Zn��OH��2+2H+��Zn2++2H2O������ZnO������������Һ�ʼ��ԣ�Ϊ�˲������µ����ʣ�����pH����ѷ���������Һ��ͨ��CO2���ʴ�Ϊ��ZnO22-+2H+=Zn(OH)2������������Ϊ��ʹZnO22-ת��ΪZn��OH��2��������������ܹ��࣬Ҫ��ֹ����Zn��OH��2+2H+��Zn2++2H2O������ZnO������CO2��
��3�������������������Ŀ������˫��ˮ�����������ӣ���Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��Fe3O4������+2����+3�������ʵ���֮��Ϊ1:2����Fe2+���ʵ���Ϊx����������ӷ���ʽ��������H2O2�����ʵ���Ϊ��![]() ����Ӧ����ԭ��Һ��Fe2+������H2O2�����ʵ���֮��Ϊx:
����Ӧ����ԭ��Һ��Fe2+������H2O2�����ʵ���֮��Ϊx:![]() =3:1���ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��3:1��
=3:1���ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��3:1��
��4����ֹFe2+[��Fe��OH��2]�����������Բ�����Ƶ�Fe3O4�������ӵĹ��̣���Ҫ�����������½��У���ҵ��ͨ��ͨ������ͨ��N2�ṩ�����������ʴ�Ϊ����ֹFe2+[��Fe��OH��2]������������ͨ��N2��
��5�����ݼ�ѹ���˵�ԭ�����������Ӱ뾶��С�����������ó��˵ķ����õ�����Fe3O4���ӣ�ԭ������������̫С������ʱ��������ֽ���ʴ�Ϊ�����ܣ���������̫С������ʱ��������ֽ��

����Ŀ����ҺX �к����±������������֣�������Ũ�Ⱦ�Ϊ��ȣ�������ˮ�ĵ���������ˮ�⣩����X �м�������ϡ���ᣬ����ɫ�������ɣ� ��������Ӧǰ������������û�б仯������˵����ȷ����
������ | Na+��Ca2+ ��Fe3+��Fe2+��Al3+��Mg2+ |
������ | OH-�� |
A.��ҺX �п�����![]() ��
��![]() �е�һ�ֻ����ֶ���
�е�һ�ֻ����ֶ���
B.��ҺX ���Ƿ���Na+����ȷ������ͨ����ɫ��Ӧ��ȷ��
C.��ҺX �п�����2 �������ӣ�3 ��������
D.ԭ��Һһ��û��Al3+��Fe 3+����Na+,��![]() ��ȷ��
��ȷ��