��Ŀ����
17��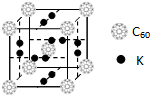 ̼Ԫ���ǹ���������������һ��Ԫ�أ��������������ǵ�����ϢϢ��أ�
̼Ԫ���ǹ���������������һ��Ԫ�أ��������������ǵ�����ϢϢ��أ���1��C60������������ϣ���֪���ʯ�е�C-C�ļ���Ϊ154.45pm��C60��C-C����Ϊ145��140pm����ͬѧ�ݴ���ΪC60���۵���ڽ��ʯ������Ϊ�Ƿ���ȷ������ȷ�������ȷ������ȷ����������C60�Ƿ��Ӿ��壬���ʯ��ԭ�Ӿ��壮
��2����ѧ�Ұ�C60�ͼز�����һ��������һ�ָ���ϩ������侧����ͼ��ʾ���������ڵ���ʱ��һ�ֳ����壮д����̬̼ԭ�ӵĵ����Ų�ͼ
 �������ʵ�Kԭ�Ӻ�C60���ӵĸ�����Ϊ3��1��
�������ʵ�Kԭ�Ӻ�C60���ӵĸ�����Ϊ3��1����3����C60��ѧ���ֺϳ���Si60��N60��C��Si��Nԭ�ӵ縺���ɴ�С��˳����N��C��Si��Si60������ÿ����ԭ��ֻ�����ڵ�3����ԭ���γɹ��ۼ�����ÿ����ԭ������㶼����8�����ȶ��ṹ����Si60������Si���ӻ�����Ϊsp2��
��4��Fe��CO��5��һ�ֳ����������ɴ������һ�Ǧ��Ϊ���͵Ŀ��������
��д��CO��һ�ֳ����ȵ�������ӵĽṹʽN��N��������ȽϷе�ߵ�ΪCO�������ʽ����
��Fe��CO��5��һ�������·�����Ӧ��Fe��CO��5��s���TFe��s��+5CO��g������֪����Ӧ�����У����ѵĻ�ѧ��ֻ����λ�����ɴ��жϸ÷�Ӧ���γɵĻ�ѧ������Ϊ��������
��5��NiXO���徧���ṹΪNaCl�ͣ����ھ���ȱ�ݣ�xֵΪ0.88�������߳�Ϊ4.28��10-10m������������Niԭ��֮�����̾���Ϊ3.03��10-10m����ȷ��0.01�����������е�Ni�ֱ�ΪNi2����Ni3�����˾�����Ni2����Ni3�������������Ϊ8��3��
���� ��1���۵��һ����ɣ�ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壻
��2�����ݵ����Ų����ɣ�д��̼ԭ�ӵ����Ų�ͼ�����ݾ����ķ���ԭ������ÿ�������ṹ�����ӵĸ�����
��3������ͬһ���ڣ�ͬһ���壬Ԫ�ص縺�Ա仯���ɣ��Ƚ�Ԫ�ص縺�Դ�С��Si�������4�����ӣ��γ�4�����õ��Ӷԣ�������8�����ȶ��ṹ��ÿ����ԭ��ֻ�����ڵ�3����ԭ���γɹ��ۼ�����ÿ��Siԭ���γ�2��������һ��˫����
��4�����ɵȵ����嶨���֪CO��һ�ֳ����ȵ�����Ϊ������COΪ���Է����۵�ȵ����ߣ�
�ڶ�����λ���������ΪCO������ԭ�ӽ�ϳɽ������壻
��5������NiXO���徧���ṹΪNaCl�ͣ����Ծ���������Niԭ��֮�����̾���Ϊ������Խ��ߵ�$\frac{1}{2}$��������Խ�������ɾ�����߳���ã�����NiXO�л��ϼ۴�����Ϊ�����þ�����Ni2����Ni3������������ȣ���
��� �⣺��1���۵��һ����ɣ�ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壬C60���ڷ��Ӿ��壬���ʯ����ԭ�Ӿ��壬���Խ��ʯ���۵����C60��
�ʴ𰸣�����ȷ��C60�Ƿ��Ӿ��壬���ʯ��ԭ�Ӿ��壻
��2��̼ԭ�ӵ�ԭ������Ϊ6��������Ų�ͼ�� �� �ھ����У�Kԭ�Ӻ�C60���ӵĸ�����Ϊ��12��2������8��8+1��=3��1��
�� �ھ����У�Kԭ�Ӻ�C60���ӵĸ�����Ϊ��12��2������8��8+1��=3��1��
�ʴ𰸣� ��3��1��
��3��1��
��3��ͬһ���ڣ������ң��縺��������ͬһ���壬���ϵ��£��縺����С����ˣ�ԭ�ӵ縺���ɴ�С��˳���ǣ�N��C��Si��Si�������4�����ӣ��γ�4�����õ��Ӷԣ�������8�����ȶ��ṹ��ÿ����ԭ��ֻ�����ڵ�3����ԭ���γɹ��ۼ�����ÿ��Siԭ���γ�2��������һ��˫������ÿ��˫����1���м���1���Ҽ���������ֻ��1���Ҽ�����ÿ��Siԭ�Ӻ���3���Ҽ�����۲���Ӷ���Ϊ3��Ϊsp2�ӻ���
�ʴ𰸣�N��C��Si��sp2��
��4����CO�ĵȵ�����Ϊ�����������ĽṹʽΪN��N��������Է���������ȵķ��Ӿ�����ԣ����Է��ӷе�ߣ�COΪ���Է��ӣ�����Ϊ�Ǽ��Է��ӣ�CO�ķе�ߣ�
�ʴ�Ϊ��N��N��CO��
�ڴ����������ԭ��Ϊ����ԭ�ӣ����ڶ��ѵ�������ԭ�Ӻ�����֮�����λ�������Զ��Ѻ������γ�CO������ԭ�Ӽ��γɽ�������Ϊ�������壬
�ʴ�Ϊ����������
��5������NiXO���徧���ṹΪNaCl�ͣ����Ծ���������Niԭ��֮�����̾���Ϊ������Խ��ߵ�$\frac{1}{2}$���ɾ�����߳�����þ�����Խ���Ϊ��Ϊ��$\sqrt{2}$��4.28��10-10m������$\frac{1}{2}$��4.28��10-10m=3.03��10-10m���辧����Ni2����Ni3�������������Ϊx��y������NiXO�л��ϼ۴�����Ϊ���֪��$\frac{2x+3y}{x+y}$��0.88=2���ɴ˽��x��y=8��3��
�ʴ�Ϊ��3.03��10-10��8��3��
���� ������Ҫ�����˵����ܡ��縺�ԡ������Ų�ͼ�����ӵĿռ乹�͡������ṹ�ļ���ȣ������漰��֪ʶ��϶࣬��Ŀ�Ѷ��еȣ������ڿ���ѧ���Ի���֪ʶ���ۺ�Ӧ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| �������� | ԭ�Ӿ��� | ���Ӿ��� | ���Ӿ��� |
| A�� | ������ | ���� | ������ |
| B�� | ������ | ̼����� | ˮ�� |
| C�� | ���ʯ | �ռ� | �� |
| D�� | �� | ���� | ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
������ԭ��Ӧ��
2FeCl3+2HI=2FeCl2+I2+2HCl�� 2Co��OH��3+6HCl=2CoCl2+Cl2��+6H2O
2Fe��OH��2+I2+2KOH=2Fe��OH��3+2KI�� 3I2+6KOH=5KI+KIO3+3H2O
���ֽⷴӦ��
2HSCN+K2CO3=2KSCN+CO2��+H2O�� KCN+CO2+H2O=HCN+KHCO3
�ȷֽⷴӦ��4NaClO $\frac{\underline{\;\;��\;\;}}{\;}$ 3NaCl+NaClO4��NaClO4 $\frac{\underline{\;\;��\;\;}}{\;}$ NaCl+2O2��
����˵����ȷ�ǣ�������
| A�� | �����ԣ�������Һ����FeCl3��Co��OH��3��I2 | B�� | ��ԭ�ԣ�������Һ����Fe��OH��2��I2��KIO3 | ||
| C�� | ���ȶ��ԣ�NaCl��NaClO��NaClO4 | D�� | ���ԣ�ˮ��Һ����H2CO3��HSCN��HCN |
| A�� | Mg3N2+H2O�T3Mg��OH��2+2NH3�� | B�� | NH3+CO2+H2O�TNH4HCO3 | ||
| C�� | 2NaOH+Cl2�TNaCl+NaClO+H2O | D�� | 2Na2O2+2CO2�T2Na2CO3+O2 |
| A�� | ��Ӧ��������Һ��pH���ף��� | |
| B�� | ��Ӧ��ʼʱ�����ʣ��ף��� | |
| C�� | ��Ӧ����ʱ�䣺�ף��� | |
| D�� | ��Ӧ��ʼʱ��������ʵ���Ũ�ȣ��ף��� |
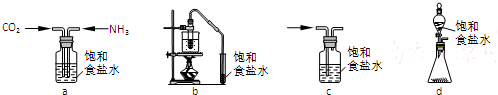
 ��ʾ�����й����л��� I��˵����ȷ����CD��
��ʾ�����й����л��� I��˵����ȷ����CD��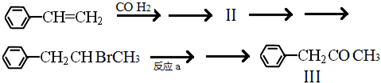
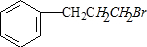 ��
�� ��
�� +NaOH$��_{��}^{H_{2}O}$
+NaOH$��_{��}^{H_{2}O}$ +NaBr��
+NaBr��