��Ŀ����
(8�֣�ÿ��2��)ˮ������֮Դ���������ǵ�����������ء��ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮҲ��һ�ֳ��õ��Լ���
(1)д����H2O���ӻ�Ϊ�ȵ��������___ ___(��1��)��
(2)ˮ�������ض����������õ�һ��H�����γ�ˮ����ԭ��(H3O��)�����ж��������̵��������������� (����)
(3)�������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ͼ(δ��˳������)������ľ���������ͬ����________(������Ӧ�ı����д)��

(4)�ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ����(����ͼ��ʾ)����֪������������51 kg/mol��������⣬ˮ���Ӽ仹���ڷ��»���(11 kJ/mol)���������������ġ����ܡ���_____ ___kJ/mol��
(1)д����H2O���ӻ�Ϊ�ȵ��������___ ___(��1��)��
(2)ˮ�������ض����������õ�һ��H�����γ�ˮ����ԭ��(H3O��)�����ж��������̵��������������� (����)
| A����ԭ�ӵ��ӻ����ͷ����˸ı� | B��������״�����˸ı� |
| C�����Ļ�ѧ���ʷ����˸ı� | D�����еļ��Ƿ����˸ı� |

(4)�ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ����(����ͼ��ʾ)����֪������������51 kg/mol��������⣬ˮ���Ӽ仹���ڷ��»���(11 kJ/mol)���������������ġ����ܡ���_____ ___kJ/mol��

��1��H2S ��2��A ��3��BC ��4��20
��1���������ͼ۵������ֱ���ȵ��ǵȵ����壬���Ժ�ˮ��Ϊ�ȵ��������H2S��
��2��ˮ��V�ͽṹ����H3O���������Σ���ԭ�ӻ���sp3�ӻ���ѡ��A�Ǵ���ģ��������ȷ�ģ���ѡA��
��3�����ݾ����ṹ��֪��A��E�ֱ����Ȼ��ơ�CO2���⡢���ʯ���ơ�����ˮ�γɵľ����Ƿ��Ӿ��壬���Դ�ѡBC��
��4�����ݽṹ��֪��ÿ��ˮ�����γ�2�������������������ǣ�51 kg/mol��11 kg/mol����2��20 kg/mol��
��2��ˮ��V�ͽṹ����H3O���������Σ���ԭ�ӻ���sp3�ӻ���ѡ��A�Ǵ���ģ��������ȷ�ģ���ѡA��
��3�����ݾ����ṹ��֪��A��E�ֱ����Ȼ��ơ�CO2���⡢���ʯ���ơ�����ˮ�γɵľ����Ƿ��Ӿ��壬���Դ�ѡBC��
��4�����ݽṹ��֪��ÿ��ˮ�����γ�2�������������������ǣ�51 kg/mol��11 kg/mol����2��20 kg/mol��

��ϰ��ϵ�д�
�����Ŀ
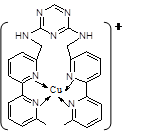



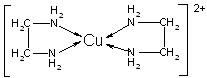
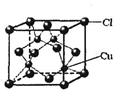
 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1��18���еĵ�7��Ԫ�ء�D��ԭ��������EС5��D��B���γ����ӻ�����侧���ṹ����ͼ��
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1��18���еĵ�7��Ԫ�ء�D��ԭ��������EС5��D��B���γ����ӻ�����侧���ṹ����ͼ��