��Ŀ����
�����������������ͻ�ѧ���ԣ����������������Ǹ�����ҵ����Ҫԭ�ϡ��ʻ����ᴿ�����漰��������Ӧ����Ϊ��
��Ni(s)��4CO(g)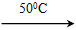 Ni(CO)4(g)��Q
Ni(CO)4(g)��Q
��Ni(CO)4(g)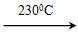 Ni(s)��4CO(g)
Ni(s)��4CO(g)
���������գ�
(1)���¶Ȳ��������£�Ҫ��߷�Ӧ����Ni(CO)4�IJ��ʣ��ɲ�ȡ�Ĵ�ʩ��____________��____________ ��
(2)����Ӧ�ڴﵽƽ����������������䣬�����¶ȣ����´ﵽƽ��ʱ_______________ ��
a��ƽ�ⳣ��K�������� b��CO��Ũ�ȼ�С
c��Ni��������С d��v��[Ni(CO)4]����
(3)�����ʻ����ᴿ�����IJ�������____________________________________________��
��(1)����COŨ�ȡ���ѹ��(2)bc
(3)�Ѵ�����CO����һ��ˮƽ���õ��ܱյIJ��������У�Ȼ���ڵ����·�Ӧ��һ��ʱ�����������һ�˼���
����

 ��У����ϵ�д�
��У����ϵ�д�25 ��ʱ�������Ϊ2 L���ܱ������У���̬A��B��C�����ʵ���n��ʱ��t�ı仯��ͼ1��ʾ����֪�ﵽƽ������¶ȣ�A��ת���ʽ�����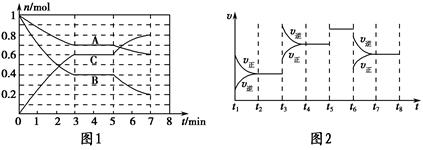
| t2��t3 | t4��t5 | t5��t6 | t7��t8 |
| K1 | K2 | K3 | K4 |
��1������ͼ1���ݣ�д���÷�Ӧ�Ļ�ѧ����ʽ��__________________���˷�Ӧ��ƽ�ⳣ������ʽK��________���ӷ�Ӧ��ʼ����һ��ƽ��ʱ��ƽ������v��A��Ϊ________��
��2����5��7 min�ڣ���Kֵ���䣬��˴����߱仯��ԭ����________________��
��3����ͼ2��ʾ�˷�Ӧ�ķ�Ӧ����v��ʱ��t�Ĺ�ϵͼ�����ε�ƽ�ⳣ�������ʾ��K1��K2��K3��K4֮��Ĺ�ϵΪ________���á�>������<���������ӣ���A��ת��������һ��ʱ����________��
��ˮú��ת���ɺϳ�����Ȼ��ϳɸ�����Ʒ��ʯ����Ʒ�ǻ����ļ�Ϊ��Ҫ������ȥˮ�������ˮú����Ҫ��H2��CO��CO2��������H2S��CH4��������ȥH2S�ɲ��ô���Ǵ�ת����������CH4ת����CO���õ�CO��CO2��H2�Ļ�����壬������ĺϳɼ״�ԭ������
(1)��ˮú������Ҫ��ѧ��Ӧ����ʽΪ��C(s)+H2O(g) CO(g)+H2(g)���˷�Ӧ�����ȷ�Ӧ��
CO(g)+H2(g)���˷�Ӧ�����ȷ�Ӧ��
�ٴ˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ ��
������������̼��ת���ʵĴ�ʩ�� ��
| A������C(s) | B������H2O(g) | C�������¶� | D������ѹǿ |
 CO(g)+2H2O(g) ��H="-519" kJ/mol����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ��(����������ͬ)
CO(g)+2H2O(g) ��H="-519" kJ/mol����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ��(����������ͬ)��X��750��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
��Y��600��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
��Z��440��ʱ��Ч����ߣ���ʹ�淴Ӧ���ʼӿ�Լ1��106����
��֪����������Ϣ������Ϊ��������Ӧ��ѡ������˴����� (�X������Y����Z��)��ѡ��������� ��
��10 L���ܱ������У��������»�ѧ��Ӧ��CO2��g����H2��g�� CO��g����H2O��g���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
CO��g����H2O��g���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
| t/�� | 700 | 800 | 830 | 1 000 | 1 200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
��ش�
��1���÷�ӦΪ ������ȡ����ȡ�����Ӧ��
��2���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK �� ��
��3����˵���÷�Ӧ�ﵽ��ѧƽ��״̬���� ������ĸ����
a��������CO2����������H2�� b��������CO����������H2O��
c��������H2����������H2O�� d��������H2����������H2��
��4��ij�¶��£���CO2��H2��0.10 mol����������У��ﵽƽ���� c��CO��="0.0080" mol��L-1����CO2��ת����Ϊ ��
��5��ij�¶��£�ƽ��Ũ�ȷ�����ʽ��c��CO2����c��H2����c��CO����c��H2O�������жϴ�ʱ���¶�Ϊ �档
�ϳɰ������Ĵ����������˹��̵�����Ҫ;�������о�������ȷ������ָ�����ϳɰ���Ӧ��ƽ�ⳣ��Kֵ���¶ȵĹ�ϵ���£�
| �� �ȣ��棩 | 360 | 440 | 520 |
| Kֵ | 0.036 | 0.010 | 0.0038 |
��1����д����ҵ�ϳɰ��Ļ�ѧ����ʽ_________________________________________��
�����ϱ����ݿ�֪�÷�ӦΪ���ȷ�Ӧ��������_____________________________________��
�������ϣ�Ϊ������ƽ��ʱH2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ�ǡ�������ţ�
a������ѹǿ b��ʹ�ú��ʵĴ���
c�������¶� d����ʱ����������е�NH3
��2��ԭ����H2��ͨ����Ӧ CH4(g) + H2O (g)
 CO(g) + 3H2(g) ��ȡ����֪�÷�Ӧ�У�����ʼ������е�
CO(g) + 3H2(g) ��ȡ����֪�÷�Ӧ�У�����ʼ������е�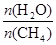 �㶨ʱ���¶ȡ�ѹǿ��ƽ������CH4������Ӱ������ͼ��ʾ��
�㶨ʱ���¶ȡ�ѹǿ��ƽ������CH4������Ӱ������ͼ��ʾ��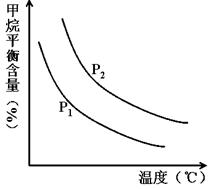
��ͼ�У��������߱�ʾѹǿ�Ĺ�ϵ�ǣ�P1________P2�����������������������
�ڸ÷�ӦΪ_____________��Ӧ������ȡ����ȡ�����
��3��ԭ����H2����ͨ����ӦCO(g) + H2O(g)
 CO2 (g) + H2(g) ��ȡ��
CO2 (g) + H2(g) ��ȡ����T ��ʱ�����ݻ��̶�Ϊ5 L�������г���1 molˮ������1 mol CO����Ӧ��ƽ����CO��Ũ��Ϊ0.08 mol��L-1����ƽ��ʱCO��ת����Ϊ______�����¶��·�Ӧ��ƽ�ⳣ��KֵΪ_________��
�ڱ����¶���ΪT �棬�ı�ˮ������CO�ij�ʼ���ʵ���֮�ȣ������������з�Ӧ�����������ܹ�˵����ϵ����ƽ��״̬����_____________������ţ���
a��������ѹǿ����ʱ��ı�
b�����������ܶȲ���ʱ��ı�
c����λʱ��������a mol CO2��ͬʱ����a mol H2
d���������n(CO) : n(H2O) : n(CO2) : n(H2) �� 1: 16 : 6 : 6
 CO2(g)��3H2(g)����H>0
CO2(g)��3H2(g)����H>0
 CH3CH2OH(g)+H2O(g)�����ܱ������г���10 mol CO��20mol H2���ڴ��������·�Ӧ�����Ҵ���CO��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3CH2OH(g)+H2O(g)�����ܱ������г���10 mol CO��20mol H2���ڴ��������·�Ӧ�����Ҵ���CO��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
 CH3OH(g)��H2O(g)������6mol CO2��8 mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����������ͼ��ʾ��ʵ�ߣ���
CH3OH(g)��H2O(g)������6mol CO2��8 mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����������ͼ��ʾ��ʵ�ߣ���

 N2O4(g)����H��0���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ��ͼ����ʾ����ش�
N2O4(g)����H��0���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ��ͼ����ʾ����ش�
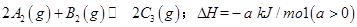 ����һ���д����Ĺ̶��ݻ��������м���2mol
����һ���д����Ĺ̶��ݻ��������м���2mol 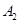 ��1mol
��1mol 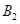 ����500��ʱ��ַ�Ӧ��ƽ���
����500��ʱ��ַ�Ӧ��ƽ���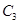 ��Ũ��Ϊw mol/L���ų�����b kJ��
��Ũ��Ϊw mol/L���ų�����b kJ�� ���������������������
���������������������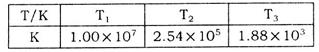
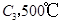 ʱ��ַ�Ӧ��ƽ�����������ckJ��
ʱ��ַ�Ӧ��ƽ�����������ckJ��