��Ŀ����
����Ŀ��ԭ�ӽṹ��Ԫ�����ڱ���Ԫ���������߹�ϵ���С�
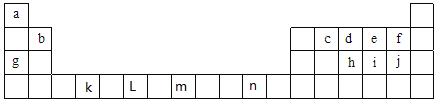
A��B��D��E��FΪԭ���������������ǰ������Ԫ�أ�����A�����������������ڲ��������2����B��D��EΪͬ����Ԫ�أ�Bԭ�ӵĺ��������������δ�ɶԵ�������5����Eԭ���������1��δ�ɶԵ��ӣ�Fԭ�Ӻ�����22���˶�״̬�ĵ��ӡ�
��ش��������⣺
��1��FԪ��λ�����ڱ�_____________������۵����Ų�ͼΪ��_____________��
��2��B��D��E����Ԫ���У���һ��������С����_____________ (��Ԫ�ط���)��д��AD2�ĵȵ�����_____________ (���Ӻ������Ӹ�дһ��)��
��3��AO2��DO2�۵�ߵ���_____________��ԭ����_____________��
���𰸡� d ![]() S CO2 ��N2O, CNO- ��SCN- SO2 SO2��CO2 ��Ϊ���Ӿ���,SO2 ��Է��������ϴ���Ϊ���Է���,���»�����
S CO2 ��N2O, CNO- ��SCN- SO2 SO2��CO2 ��Ϊ���Ӿ���,SO2 ��Է��������ϴ���Ϊ���Է���,���»�����
��������A��B��D��E��FΪԭ���������������ǰ������Ԫ�أ�A�����������������ڲ��������2����ΪCԪ����B��D��EΪͬ����Ԫ�أ�Bԭ�ӵĺ��������������δ�ɶԵ�������5������B�ĺ�������Ų�ʽΪ1s22s22p63s23p3������BΪPԪ����Eԭ���������1��δ�ɶԵ��ӣ���EΪClԪ����Dֻ��ΪSԪ�أ�Fԭ�Ӻ�����22���˶�״̬�ĵ�����˵��Fԭ�Ӻ�����22�����ӣ���FΪTiԪ����
(1). FΪTiԪ�أ�λ�����ڱ��е�4���ڣ���IVB�壬�����ڱ�������d��Ԫ�أ���۵����Ų�ʽΪ3d24s2����۵����Ų�ͼΪ![]() ���ʴ�Ϊ��d��
���ʴ�Ϊ��d��![]() ��
��
(2). ͬ��������Ԫ�أ�����ԭ������������һ�����ܳ�����������ƣ����ǵڢ�A��͵�VA��Ԫ�ط���������P��S��Cl����Ԫ���У���һ��������С����S���ȵ�������ָԭ�Ӹ�����ͬ���۵���������ͬ����������CS2��Ϊ�ȵ�������У�CO2��N2O��CNO����SCN�����ʴ�Ϊ��S��CO2��N2O��CNO����SCN����
��3��AO2ΪCO2��DO2ΪSO2�����߾�Ϊ���Ӿ��壬��Է�������Խ���Ӽ�������Խ���۷е�Խ�ߣ������۵�ߵ���SO2���ʴ�Ϊ��SO2��SO2��CO2��Ϊ���Ӿ��壬SO2��Է��������ϴ���Ϊ���Է��ӣ����»�����

����Ŀ������ʵ���������ʵ��Ԥ��ʵ��Ŀ�Ļ����ý��۶�Ӧ��ȷ����(����)
ѡ�� | ʵ���������ʵ | ʵ��Ŀ�Ļ���� |
A | ����ɫ��Һ | ˵��ԭ��Һ��һ��������FeCl3 |
B | CaO | ����ʯ���Ʊ�NaOH��Һ |
C | ���ռ������� | ������һ������SO42- |
D | H3PO3+2NaOH(����)=Na2HPO3+2H2O | H3PO3������Ԫ�� |
A. A B. B C. C D. D