��Ŀ����
����Ŀ���о�����CO��N2O��Fe+�����·�����Ӧ�������仯����Ӧ������ͼ��ʾ��������Ӧ�քeΪ����N2O+Fe+=N2+FeO (��������FeO++CO=CO2+Fe+ (�죩������˵����ȷ����

A. ��Ӧ����������ԭ��Ӧ����Ӧ���Ƿ�������ԭ��Ӧ
B. ������Ӧ��Ϊ���ȷ�Ӧ���ܷ�Ӧ�Ļ�ѧ��Ӧ�����ɷ�Ӧ�ھ���
C. Fe+ʹ��Ӧ�Ļ�ܼ�С��FeO+���м����
D. ��ת��lmol���ӣ�������II.2LN2O
���𰸡�C
��������
A.��Ӧ�����ھ���Ԫ�ػ��ϼ۵���������˶���������ԭ��Ӧ��A����
B.��ͼ��֪��Fe++N2O��FeO++N2��FeO++CO��Fe++CO2�����о�Ϊ��Ӧ������������������������������������Ӧ���Ƿ��ȷ�Ӧ���ܷ�Ӧ�Ļ�ѧ��Ӧ�������������ķ�Ӧ�پ�����B����
C. Fe+��������ʹ��Ӧ�Ļ�ܼ�С��FeO+�Ƿ�Ӧ�����в��������ʣ�������м���C��ȷ��
D.����û��ָ��������������Բ���ȷ������������D����
�ʺ���ѡ����C��

����Ŀ��(1)�Լ״�Ϊԭ����ȡ�ߴ�H2������Ҫ��Ӧ�ü�ֵ���״�ˮ��������������Ҫ��������������Ӧ��
����Ӧ��![]() H=+49kJmol-1
H=+49kJmol-1
����Ӧ��![]() H=+41kJmol-1
H=+41kJmol-1
���״������ڴ����������ѽ�ɵõ�H2��CO����÷�Ӧ���Ȼ�ѧ����ʽΪ_________________�����ܼӿ췴Ӧ�����������CH3OHƽ��ת���ʵ�һ�ִ�ʩ��______________��
�������ʵ�����ˮ����![]() �Լ״�ˮ������������ĺô���__________��
�Լ״�ˮ������������ĺô���__________��
��ij�¶��£���n(H2O)��n(CH3OH)=1��1��ԭ������������ܱ������У���ʼѹǿΪP1����Ӧ��ƽ��ʱ��ѹǿΪP2����ƽ��ʱ�״���ת����Ϊ________________(���Ը���Ӧ���ú�P1��P2��ʽ�ӱ�ʾ)��
(2)��ҵ����CH4��ˮ������һ����������ȡH2��ԭ��Ϊ��![]() H=+203kJmol-1
H=+203kJmol-1
���÷�Ӧ�淴Ӧ���ʱ���ʽΪ��v��=k��c(CO)��c3(H2)��kΪ���ʳ�������ij�¶��²��ʵ���������±���
c(CO)/mol��L��1 | c(H2)/mol��L��1 | v��/mol��L��1��min��1 |
0.05 | c1 | 4.8 |
c2 | c1 | 19.2 |
c2 | 0.15 | 8.1 |
���������ݿɵø��¶��£��÷�Ӧ���淴Ӧ���ʳ���kΪ_________L3��mol��3��min��1��
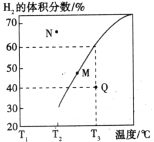
�������Ϊ3L���ܱ�������ͨ�����ʵ�����Ϊ3mol��CH4��ˮ��������һ�������·���������Ӧ�����ƽ��ʱH2������������¶ȹ�ϵ��ͼ��ʾ��N��v��____________M��v��(��������������С����)��Q���Ӧ�¶��¸÷�Ӧ��ƽ�ⳣ��K=_______________mol2��L��2��ƽ������������м���1molCH4��1molCO��ƽ����_____________�����ƶ�(��������Ӧ�������淴Ӧ��)��
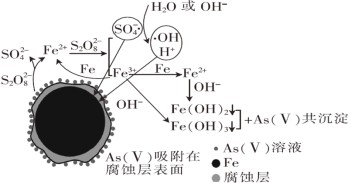
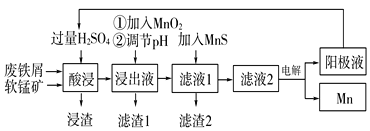
����Ŀ��������ij����С�����÷���м��ԭ�������̿�(��Ҫ�ɷ�ΪMnO2)�Ʊ������̼��������Һ���̵Ĺ�������ͼ��
��֪���ٽ���Һ����Ҫ����Fe3����Fe2����Co2����Ni2�������ʽ������ӣ�
���������������pH���±���
���� | Fe(OH)2 | Fe(OH)3 | Ni(OH)2 | Co(OH)2 | Mn(OH)2 |
��ʼ������pH | 7.5 | 2.7 | 7.7 | 7.6 | 8.3 |
��ȫ������pH | 9.7 | 3.7 | 8.4 | 8.2 | 9.8 |
��ش��������⣺
��1���������ǰ��ԭ�Ϸ����Ŀ����____��
��2������ͼ�С��ټ���MnO2��������____��MnO2�������������Լ�____(�ѧʽ)���档
��3������ͼ�С��ڵ���pH�����Գ�ȥij�ֽ������ӣ�Ӧ����ҺpH���ڿ��Ƶķ�Χ��___��7.6�����������У���ѭ��ʹ�õ�һ��������___(�ѧʽ)��
��4������Һ���м���MnS�������dz�ȥCo2����Ni2�������ӣ����п��Է�����ӦΪMnS(s)��Ni2��(aq)=NiS(s)��Mn2��(aq)�ȡ����÷�Ӧ��ȫ����Һ2�е�Mn2����Ni2�������ʵ���Ũ��֮����___[��֪Ksp(MnS)��2.8��10��10��Ksp(NiS)��2.0��10��21]��
��5�����ʵ������£���MnSO4��H2SO4��H2OΪ��ϵ�ĵ��Һ�е��Ҳ�ɻ��MnO2���������缫��ӦʽΪ____��
��6����״��������Ԫ���Ͽ���Ϊ����ӵ���������ϣ��仯ѧʽΪLiNixCoyMnzO2������Ni��Co��Mn�Ļ��ϼ۷ֱ�Ϊ+2��+3��+4����x=y=![]() ʱ��z=___��
ʱ��z=___��