��Ŀ����
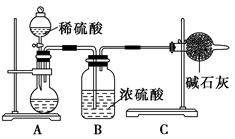
Ϊ��̽��SO2��Na2O2�ķ�Ӧ�Ƿ�������CO2��Na2O2�ķ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã��ش��������⣺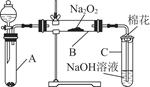
��1���ƿ������������ǵ�ľ������C�Թܿڣ�δ��ľ����ȼ����ͬѧ�����ΪSO2��Na2O2�ķ�Ӧ��ͬ��CO2���밴��ͬѧ�Ĺ۵�д����Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ��Ϊ���۷�Ӧԭ����Σ����ն���O2��������ͬѧ�������� ��������ͬѧ�Ĺ۵㣬��װ�������ĸĽ���
��
��3������Na2O2��ȫ��Ӧ����Ӧ��Bװ���й�������������ǣ���Na2SO3����Na2SO4����Na2SO3��Na2SO4��
�����ʵ�鷽�����飬д��ʵ�鲽���Լ�Ԥ������ͽ��ۣ�����±���
��ѡ�Լ���2 mol��L��1 HCl��Һ��1 mol��L��1 HNO3��Һ��1 mol��L��1 BaCl��Һ��1 mol��L��1 Ba��NO3��2��Һ��0.01 mol��L��1 KMnO4������Һ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡB�е�����������Ʒ���Թ��У��μ���������ˮ���ܽ⣬Ȼ��ȡ��������Һ�ֱ����ڢ��Թ��� | ������ȫ�ܽ� |
| ����2�������Թ��м��� ���ٵμ� | �� |
| ��֤���������к�Na2SO4 | |
| ����3�������Թ��� | |
| | �� �� |
| ��֤������������Na2SO3���� | |
| | |
| ��˵����������û��Na2SO3�� | |
��4�����������������ƺ����IJⶨ��
��ȡa g���������Ƴ�100 mL��Һ��ȡ10.00 mL����Һ����ƿ�У����뼸�ε�����Һ��ָʾ������0.010 0 mol��L��1��ˮ���еζ����ζ��յ�����Ϊ ����¼���ݣ��ظ��ζ�2�Σ�ƽ�����ĵ�ˮ20.00 mL��
�ڼ��㣺���������������Ƶ���������Ϊ ��
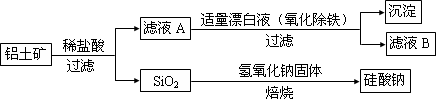
��1��SO2��Na2O2=Na2SO4
��2��A�����ɵ�SO2�����к���ˮ����
��A��B֮������һ��װ��Ũ�����ϴ��ƿ�����������ʵĸ���װ�ã�
��3��
��4������Һ������ɫ���Ұ�����ڲ���ɫ����2�������Թ��м���������1_mol��L��1�������ٵμ�1_mol��L��1_BaCl2��Һ �а�ɫ������������֤���������к�Na2SO4 ����3�������Թ�������2��3��0.01_mol��L��1_KMnO4������Һ���� ��KMnO4��Һ�Ϻ�ɫ��ȥ����֤������������Na2SO3�� ��KMnO4��Һ�Ϻ�ɫ����ȥ����˵����������û��Na2SO3
�� ��100%
��100%
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д����ǻ��õļ����Ԫ�أ��Ƽ��仯�������������������й㷺��Ӧ�á�
������м��㣺
��1���������ƣ�NaN3����ײ���ֽ�����ƺ͵������ʿ�Ӧ����������ȫ���ҡ���78�˵���������ȫ�ֽ⣬������״���µ���___________________L ��
��2����-�غϽ���ں˷�Ӧ���������Ƚ���Һ��5.05 g��-�غϽ�����200 mLˮ����0.075 mol������������Һ���������Ƶ����ʵ���Ũ��______________________������Һ������仯����
��3������������Һ�����������ˣ��õ��������Ƶ���Һ�������Һ��ͨ�������̼�������з�Ӧ�� 2NaAl(OH)4+CO2��2Al(OH)3��+Na2CO3+H2O����֪ͨ�������̼112 L����״���£������ɵ�Al(OH)3��Na2CO3�����ʵ���֮��Ϊ4:5���������Һ��ͨ��Ķ�����̼Ϊ224L����״���£����������ɵ� Al(OH)3��Na2CO3�����ʵ��������ֵ��
��4��Ϊ�ⶨij�������պ�������������������е�Ԫ�ص������������ֽ���ͬ��������ι���ֱ���뵽50.00mL��ͬŨ�ȵ�����������Һ�У���ˮԡ����������ȫ���ݳ�(���¶�����β��ֽ�)�������徭�������Ũ����������ȫ���ⶨŨ�������ӵ����������ֲⶨ������±���
| ������/g | 10.00 | 20.00 | 30.00 | 50.00 |
| Ũ�������ӵ�����/g | m | m | 1.29 | 0 |
�Իش�
����εijɷ�Ϊ_______________________________��
������е�Ԫ�ص���������Ϊ��_______________________________(����ʽ���㣩��