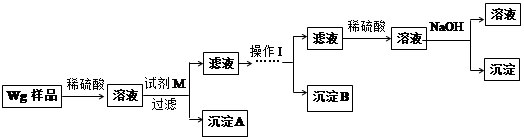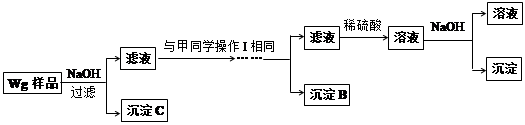题目内容
Ⅰ.铁是人体必需的微量元素,绿矾(FeSO4·7H2O)是治疗缺铁性贫血药品的重要成分。
(1)FeSO4溶液在空气中会因氧化变质产生红褐色沉淀,其发生反应的离子方程式是 ;实验室在配制FeSO4溶液时常加入 以防止其被氧化。请你设计一个实验证明FeSO4溶液是否被氧化 。
Ⅱ.硫酸亚铁铵[(NH4)2Fe(SO4)2·6H2O]较硫酸亚铁不易被氧气氧化,常用于代替硫酸亚铁。
(2)硫酸亚铁铵不易被氧化的原因是 。
(3)为检验分解产物的成分,设计如下实验装置进行实验,加热A中的硫酸亚铁铵至分解完全。
①A中固体充分加热较长时间后,通入氮气,目的是 。
②装置B中BaCl2溶液的作用是为了检验分解产物中是否有SO3气体生成,若含有该气体,观察到的现象为 。
③实验中,观察到C中有白色沉淀生成,则C中发生的反应为 (用离子方程式表示)。
Ⅰ.(1)12Fe2++3O2+6H2O=Fe(OH)3↓+8Fe3+(其他合理答案也给分) 铁粉 取少量FeSO4溶液于试管中,加数滴KSCN溶液,如果溶液变红,则说明FeSO4溶液已被氧化,如不变红,则说明FeSO4溶液没有被氧化。
Ⅱ.(2)硫酸亚铁铵溶液中N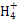 发生水解增大c(H+),抑制了Fe2+氧化反应的进行。
发生水解增大c(H+),抑制了Fe2+氧化反应的进行。
(3)①使分解产生的气体在B、C中被吸收充分
②溶液变浑浊(或出现白色沉淀)
③SO2+H2O2+Ba2+=aSO4↓+2H+(或SO2+H2O2=H++S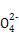 、S
、S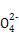 +Ba2+=aSO4↓)
+Ba2+=aSO4↓)
解析

 阅读快车系列答案
阅读快车系列答案某化学小组在学习元素周期律后,对教材中Fe2+氧化为Fe3+的实验进一步思考,并提出问题:Cl2能将Fe2+氧化为Fe3+,那么Br2和I2能否将Fe2+氧化为Fe3+?
环节一:理论推测
部分同学认为Br2和I2都可能将Fe2+氧化为Fe3+,他们思考的依据是 。
部分同学认为Br2和I2都不能将Fe2+氧化为Fe3+,还有同学认为Br2能将Fe2+氧化为Fe3+而I2不能。他们思考的依据是从上到下卤素单质氧化性减弱。
环节二:设计实验进行验证
在大试管中加适量铁粉,加入10 mL稀盐酸,振荡试管,充分反应后,铁粉有剩余,取上层清液进行下列实验。
实验1:
| 试管 | 操作 | 现象 |
| ① | 先向试管中加入2 mL FeCl2溶液,再滴加少量红棕色的溴水,振荡试管 | 溶液为黄色 |
| ② | 先向试管中加入2 mL FeCl2溶液,再滴加少量棕黄色的碘水,振荡试管 | 溶液为黄色 |
(1)同学甲认为①中现象说明溴水能将Fe2+氧化,离子方程式为 。
同学乙认为应该补充实验,才能得出同学甲的结论。请你帮助同学乙完成实验:
实验2:
| 操作 | 应该观察到的现象 |
| | |
(2)该小组同学对②中溶液呈黄色的原因展开了讨论:
可能1:碘水与FeCl2溶液不反应,黄色是碘水稀释后的颜色。
可能2: 。
实验3:进行实验以确定可能的原因。
| 操作 | 现象 |
| 向试管②所得溶液中继续加入0.5 mL CCl4,充分振荡,静置一段时间后。取出上层溶液,滴加KSCN溶液 | 静置后,上层溶液几乎无色,下层溶液为紫色;上层溶液滴加KSCN溶液后,出现浅红色 |
同学丙认为该实验现象可以说明是“可能2”成立,同学丁认为不严谨,于是设计了实验4:
实验4:
| 操作 | 现象 |
| 向另一支试管中加入2 mL FeCl2溶液,滴加0.5 mL碘水后,再加入0.5 mL乙酸乙酯,充分振荡,静置一段时间后。取出下层溶液,滴加KSCN溶液 | 静置后,上层液为紫色,下层液几乎无色;下层溶液滴加KSCN溶液后,没有出现浅红色 |
你认为实验4设计的主要目的是 。
同学丁根据实验4现象得出结论:在本次实验条件下,碘水与FeCl2溶液反应的程度很小。
(3)Cl2、Br2、I2氧化Fe2+的能力逐渐减弱,用原子结构解释原因: 。
为了探究SO2与Na2O2的反应是否类似于CO2与Na2O2的反应,甲同学设计了如图所示的实验装置,回答下列问题: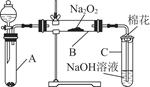
(1)移开棉花,将带火星的木条放在C试管口,未见木条复燃,甲同学因此认为SO2与Na2O2的反应不同于CO2。请按甲同学的观点写出反应的化学方程式 。
(2)乙同学认为无论反应原理如何,最终都有O2产生,乙同学的理由是 。按照乙同学的观点,该装置需做的改进是
。
(3)假设Na2O2完全反应,反应后B装置中固体生成物可能是:①Na2SO3;②Na2SO4;③Na2SO3和Na2SO4。
请设计实验方案检验,写出实验步骤以及预期现象和结论,完成下表:
限选试剂:2 mol·L-1 HCl溶液,1 mol·L-1 HNO3溶液,1 mol·L-1 BaCl溶液,1 mol·L-1 Ba(NO3)2溶液,0.01 mol·L-1 KMnO4酸性溶液。
| 实验步骤 | 预期现象和结论 |
| 步骤1:取B中的少量固体样品于试管中,滴加足量蒸馏水,溶解,然后取少量待测液分别置于Ⅰ、Ⅱ试管中 | 固体完全溶解 |
| 步骤2:往Ⅰ试管中加入 ,再滴加 | , |
| 则证明生成物中含Na2SO4 | |
| 步骤3:往Ⅱ试管中 | |
| | 若 , |
| 则证明生成物中有Na2SO3;若 | |
| | |
| 则说明生成物中没有Na2SO3。 | |
(4)生成物中亚硫酸钠含量的测定:
①取a g生成物配制成100 mL溶液,取10.00 mL该溶液于锥形瓶中,加入几滴淀粉溶液作指示剂,用0.010 0 mol·L-1碘水进行滴定,滴定终点现象为 。记录数据,重复滴定2次,平均消耗碘水20.00 mL。
②计算:生成物中亚硫酸钠的质量分数为 。

 、
、 

 样品含有少量
样品含有少量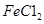 杂质。现要测定其中
杂质。现要测定其中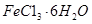 的质量分数,实验按以下步骤进行:
的质量分数,实验按以下步骤进行: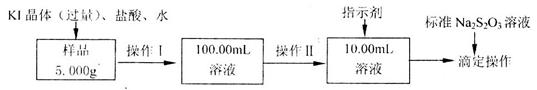
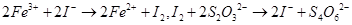
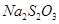 溶液18.00mL。该样品中
溶液18.00mL。该样品中