��Ŀ����
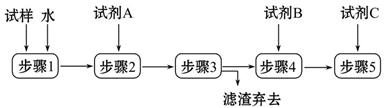
ijУ��ѧ����С��Ϊ�˼���̼���ƺ�̼������������ɫ���壬�ò�ͬ�ķ�����������ʵ�飬��ͼI������ʾ��
��1��ֻ����ͼI������ʾʵ�飬�ܹ��ﵽʵ��Ŀ�ĵ��� ����װ����ţ���
��2��ͼI��ʾʵ��ǰҪ�ȼ��������ԣ�����������Եķ����ǣ��رշ�Һ©�������������ܿڽ���ˮ�У� �������������á�
��3��ͼ����ʾʵ����ܼ������������ʣ����Ƚ�ͼ���ͼ������ʵ�飬ͼ��ʵ����ŵ��� ��
��4������ͼ��ʵ����֤̼���ƺ�̼�����Ƶ��ȶ��ԣ����Թ�B��װ��Ĺ�������� ���ѧʽ����
��5����ȥ̼���ƹ���������̼�����Ƶķ����� ���ѧ����ʽ����
��1��ͼ����1�֣�
��2����˫����ס����Թܣ����ܿ�������ð�����ɿ��ֺ��ܿ��γ�һˮ������2�֣�ÿ�����ֵ��1�֣�
��3������ͬʱ���жԱ�ʵ�飬�����ڱȽϷ�������1�֣��ӶԱ�ʵ��ĽǶȻش��֣�
��4��NaHCO3����1�֣�
��5��2NaHCO3 Na2CO3��CO2����H2O ����2�֣�
Na2CO3��CO2����H2O ����2�֣�
���������������1��Na2CO3��NaHCO3������ϡ���ᷴӦ����������ʹ����ʯ��ˮ����ǵ����壬��I�������ڶ��ߵļ���Na2CO3��NaHCO3��ϡ���ᷴӦ������ͬ�����߱�ǰ�߷�Ӧ��ö࣬II���������͵����ʲ�ͬ�������ڼ�����ߣ���2���������Լ�鳣�÷�����֪���رշ�Һ©�������������ܿڽ���ˮ�У���˫����ס����Թܣ����ܿ�������ð�����ɿ��ֺ��ܿ��γ�һˮ����˵��װ�������Ժã���3��ͼIIIֻ���Ⱥ����Na2CO3��NaHCO3����ʱ���ȶ��ԣ���ͼIV�����ͬʱ���жԱ�ʵ�飬�����ڱȽϷ�������4�������¶ȣ�A>B�������֮�зֱ�ӦʢNa2CO3��NaHCO3����5��Na2CO3���Ȳ���ֽ⣬2NaHCO3 Na2CO3��CO2����H2O��
Na2CO3��CO2����H2O��
���㣺����Na2CO3��NaHCO3�����ʵļ�����ᴿ�����֪ʶ��

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
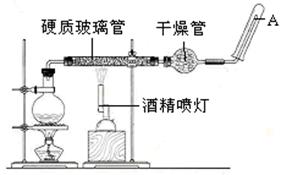
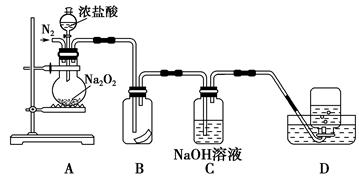
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�Ϊ��̽��SO2��Na2O2�ķ�Ӧ�Ƿ�������CO2��Na2O2�ķ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã��ش��������⣺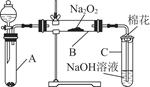
��1���ƿ������������ǵ�ľ������C�Թܿڣ�δ��ľ����ȼ����ͬѧ�����ΪSO2��Na2O2�ķ�Ӧ��ͬ��CO2���밴��ͬѧ�Ĺ۵�д����Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ��Ϊ���۷�Ӧԭ����Σ����ն���O2��������ͬѧ�������� ��������ͬѧ�Ĺ۵㣬��װ�������ĸĽ���
��
��3������Na2O2��ȫ��Ӧ����Ӧ��Bװ���й�������������ǣ���Na2SO3����Na2SO4����Na2SO3��Na2SO4��
�����ʵ�鷽�����飬д��ʵ�鲽���Լ�Ԥ������ͽ��ۣ�����±���
��ѡ�Լ���2 mol��L��1 HCl��Һ��1 mol��L��1 HNO3��Һ��1 mol��L��1 BaCl��Һ��1 mol��L��1 Ba��NO3��2��Һ��0.01 mol��L��1 KMnO4������Һ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡB�е�����������Ʒ���Թ��У��μ���������ˮ���ܽ⣬Ȼ��ȡ��������Һ�ֱ����ڢ��Թ��� | ������ȫ�ܽ� |
| ����2�������Թ��м��� ���ٵμ� | �� |
| ��֤���������к�Na2SO4 | |
| ����3�������Թ��� | |
| | �� �� |
| ��֤������������Na2SO3���� | |
| | |
| ��˵����������û��Na2SO3�� | |
��4�����������������ƺ����IJⶨ��
��ȡa g���������Ƴ�100 mL��Һ��ȡ10.00 mL����Һ����ƿ�У����뼸�ε�����Һ��ָʾ������0.010 0 mol��L��1��ˮ���еζ����ζ��յ�����Ϊ ����¼���ݣ��ظ��ζ�2�Σ�ƽ�����ĵ�ˮ20.00 mL��
�ڼ��㣺���������������Ƶ���������Ϊ ��
�ҹ�����ר�Һ�°����ĺ����Ƽ�Ļ�ѧԭ���ǽ�������̼ͨ�백ˮ���Ȼ��Ʊ�����Һ�У��仯ѧ��Ӧ����ʽΪ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��
��1����ʵ��������������ԭ���ӷ�Ӧ������Һ�з����̼�����ƾ��壬Ӧѡ������װ���е� ��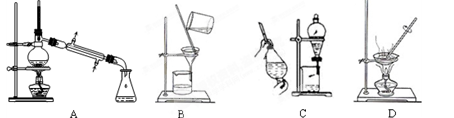
��2��ʵ������̼�����ƾ����У����ܺ��е�����������Cl����NH4+��ʵ���Ҽ���Cl����ѡ�õ��Լ��ǡ���������һ���������ӵķ����� ������ţ���
| A����ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| B��������������Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| C��������������Һ�����ȣ������̪�Լ� |
| D��������������Һ�����ȣ�������ɫʯ���Լ� |
 Na2O2��
Na2O2�� ��Ʒ��������
��Ʒ��������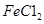 ���ʡ���Ҫ�ⶨ����
���ʡ���Ҫ�ⶨ����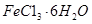 ������������ʵ�鰴���²�����У�
������������ʵ�鰴���²�����У�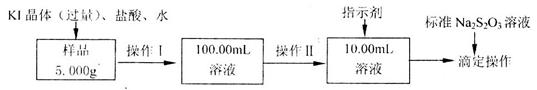
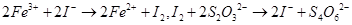
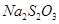 ��Һ18��00mL������Ʒ��
��Һ18��00mL������Ʒ��