��Ŀ����
17��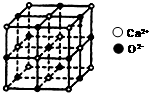 ��1��AlN�ľ���ṹ�뵥�������ƣ���AlN�����У�ÿ��Alԭ����4��Nԭ����������NԪ��ͬ�����Ԫ��Asλ��Ԫ�����ڱ��ĵ������ڣ����̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3��
��1��AlN�ľ���ṹ�뵥�������ƣ���AlN�����У�ÿ��Alԭ����4��Nԭ����������NԪ��ͬ�����Ԫ��Asλ��Ԫ�����ڱ��ĵ������ڣ����̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3����2��AԪ�ص�ԭ�Ӽ۵����Ų�Ϊns2np2��BԪ�ص�����������������Ӳ�����3����CԪ��ԭ�ӵ�M���Ӳ�p�Dz�����3��δ�ɶԵ��ӣ�DԪ��ԭ�Ӻ����M����ֻ��2�ԳɶԵ��ӣ�
�ٵ�n=2ʱ��AB2���ڷǼ��Է��ӣ�����ԡ��Ǽ��ԡ�����
����AԪ�ص�ԭ�Ӽ۵����Ų�Ϊ3s23p2��A��C��D����Ԫ�صĵ�һ�������ɴ�С��˳����P��S��Si����Ԫ�ط��ű�ʾ��
��3��CaO��������ͼ��ʾ����Ca2+����λ��Ϊ6��CaO�����NaCl����������Ų���ʽ��ͬ��CaO������۵����NaCl������۵����Ҫԭ���ǽṹ���ƣ����Ӻ˼�������������£�CaO�е�����������ɸ��࣬��������ǿ���۵���ߣ�
���� ��1�����������Ϣ��AlN�ľ���ṹ�뵥�������ơ������ݾ����Ľṹ��������NԪ��ͬ�����Ԫ��Asλ��Ԫ�����ڱ��ĵ������ڣ���ԭ������Ϊ33��������33����������������Ϊ5��
��2��A��B��C��DΪǰ������Ԫ�أ�BԪ�ص�����������������Ӳ�����3��������������������8��������BԪ��ΪOԪ�أ�CԪ��ԭ�ӵ�M���Ӳ��P�Dz�����3��δ�ɶԵ��ӣ���C��PԪ�أ�DԪ��ԭ�Ӻ����N����ֻ��2�ԳɶԵ��ӣ���D�ĺ�������Ų�ʽΪ��1s22s22p63s23p4����D��SԪ�أ�
�ٵ�n=2ʱ��A��CԪ�أ���AB2��CO2�����ݶ�����̼����������������Ƿ��غ��жϣ�
����AԪ�ص�ԭ�Ӽ۵����Ų�Ϊ3s23p2����AΪSiԪ�أ�ͬ���ڴ����ҵ�һ�����������ǵ�VA���VIA�巴����
��3���Ը�����Ϊ���ģ���X��Y��Z��������и�Ӷ�ȷ����������λ�������Ӿ�������Ӱ뾶ԽС�����Խ�࣬������Խ���۵�Խ�ߣ�
��� �⣺��1�����������Ϣ��AlN�ľ���ṹ�뵥�������ơ����������ÿ��Siԭ���γ�4�����ۼ���������4����ԭ����������ÿ��Alԭ����4��Nԭ����������NԪ��ͬ�����Ԫ��Asλ��Ԫ�����ڱ��ĵ������ڣ���ԭ������Ϊ33��������33����������������Ϊ5�����̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3��
�ʴ�Ϊ��4��1s22s22p63s23p63d104s24p3��
��2��A��B��C��DΪǰ������Ԫ�أ�BԪ�ص�����������������Ӳ�����3��������������������8��������BԪ��ΪOԪ�أ�CԪ��ԭ�ӵ�M���Ӳ��P�Dz�����3��δ�ɶԵ��ӣ���C��PԪ�أ�DԪ��ԭ�Ӻ����N����ֻ��2�ԳɶԵ��ӣ���D�ĺ�������Ų�ʽΪ��1s22s22p63s23p4����D��SԪ�أ�
�ٵ�n=2ʱ��A��CԪ�أ���AB2��CO2��������̼��ֱ���ͽṹ��������������غϣ������ǷǼ��Է��ӣ�
�ʴ�Ϊ���Ǽ��ԣ�
����AԪ�ص�ԭ�Ӽ۵����Ų�Ϊ3s23p2����AΪSiԪ�أ�ͬ���ڴ����ҵ�һ�����������ǵ�VA���VIA�巴�������һ�����ܣ�P��S��Si��
�ʴ�Ϊ��P��S��Si��
��3���Ը�����Ϊ���ģ���X��Y��Z��������и���ͼƬ֪�������ӵ���λ����6�����Ӿ�������Ӱ뾶ԽС�����Խ�࣬������Խ���۵�Խ�ߣ��ṹ���ƣ����Ӻ˼�������������£�CaO�е�����������ɸ��࣬��������ǿ���۵���ߣ�
�ʴ�Ϊ��6���ṹ���ƣ����Ӻ˼�������������£�CaO�е�����������ɸ��࣬��������ǿ���۵���ߣ�
���� ������Ҫ�����˵����Ų�ʽ��ԭ�ӽṹ����һ�����ܡ������ļ����֪ʶ������ؼ���Ҫ��������Ԫ�������ɺ����ڱ���Ԫ�ص����࣬��Ŀ�Ѷ��еȣ�ע��������Ӿ����۷е�ȽϷ�����

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�| A�� |  ��ͼװ����ȡ��ˮ�е��壬������ı���Һ���¿ڷų� | |
| B�� |  ��ͼװ�ÿ�����֤�����鷢������ȥ��Ӧ | |
| C�� |  ��ͼװ������������Һ | |
| D�� |  ��ͼװ�ÿ�˵��ŨH2SO4������ˮ�ԡ�ǿ�����ԣ�SO2����Ư���ԡ���ԭ�� |
| A�� | ��Ӧ��ϵ����ѹ�㶨 | B�� | A��B��Ũ����� | ||
| C�� | c��A����c��B��=1��3 | D�� | 2v��B����=3v��C���� |
| A�� | ��ԭ��Ӧ | B�� | ˮ�ⷴӦ | C�� | ��ȥ��Ӧ | D�� | �ӳɷ�Ӧ |
| A�� | �����������ᷴӦ���������Ƴɺ�ɫͿ�ϻ����� | |
| B�� | �̻���Ѥ���ͷ��Ǻ��ء��ơ��ơ�ͭ�Ƚ���Ԫ�صĻ�����Ļ�ѧ���ʵ�չ�� | |
| C�� | ҽ�þƾ����õ�����ֲ�ᆳ�ǻ��ٷ��;������Ƴɣ�Ũ��ͨ����75% | |
| D�� | ά����C��ˮ����ά���أ�����ǿ����ֿ������п��������� |
| A�� | 1.0L1.0mo1•L-1��NaAlO2ˮ��Һ�к��е���ԭ����Ϊ2NA | |
| B�� | ���³�ѹ�£�8gO2����4NA������ | |
| C�� | 25��ʱpH=13��NaOH��Һ�к���OHһ����ĿΪ0.1NA | |
| D�� | ���³�ѹ�£�14g��N2��CO��ɵĻ�����庬�е�ԭ����ĿΪNA |
| A�� | 2H2��g��+O2��g��=2H2O��l������H=-484kJ/mol | B�� | H2O��g��=H2��g��+$\frac{1}{2}$O2��g������H=+242kJ/mol | ||
| C�� | 2H2��g��+O2��g��=2H2O��g������H=-484kJ/mol | D�� | H2��g��+$\frac{1}{2}$O2��g��=H2O��g������H=+242kJ/mol |
| A�� | ���ͬλ��������ԭ�ӵķ��ţ�13H | |
| B�� | Mg2+�Ľṹʾ��ͼ�� | |
| C�� | �����ĵ���ʽ�� | |
| D�� | Ca��ClO��2�ĵ��뷽��ʽ��Ca��ClO��2=Ca2++2ClO- |
| A�� | �ܱ������У�9.6 g�����11.2 g���ۻ�ϼ�������17.6 g������ʱ���ų�19.12 kJ��������Fe��s��+S��s���TFeS��s����H=-95.6 kJ•mol-1 | |
| B�� | ϡ������0.1 mol•L-1 NaOH��Һ��Ӧ��H+��aq��+OH-��aq��=H2O��l����H=-57.3 kJ•mol-1 | |
| C�� | ��֪1 mol������ȫȼ������Һ̬ˮ���ų�������Ϊ285.5 kJ����ˮ�ֽ���Ȼ�ѧ����ʽΪ2H2O��l���T2H2��g��+O2��g����H=+285.5 kJ•mol-1 | |
| D�� | ��֪2C��s��+O2��g��=2CO��g����H=-221 kJ•mol-1����C��ȼ���ȡ�H=-110.5 kJ•mol-1 |