��Ŀ����
��֪A��B��C��D��E��F��G����Ԫ�����ڱ��ж���������Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�D3B�����������Ӿ�����ͬ�ĵ��Ӳ�ṹ��B��C���ɷֱ���A�γ�10�����ӷ��ӣ�B��C��ͬһ���ڣ����߿����γ������ֹ��ۻ����C��F��ͬһ���壬Bԭ��������Ӳ��p�ܼ��ϵĵ��Ӵ��ڰ���״̬��C���������������ڲ��������3����E���������������ڲ��1�����þ����Ԫ�ػش��������⣺
��1��EԪ��ԭ�ӻ�̬�����Ų�ʽ ��
��2���õ����Ų�ͼ��ʾFԪ��ԭ�ӵļ۵��ӹ��� ��
��3��F��GԪ�ض�Ӧ����ۺ����������Խ�ǿ�ķ���ʽΪ ��
��4�����Ӱ뾶D+ B3������һ������B C���縺��C F
���<������>����=������
��5��A��C�γɵ�һ����ɫ������X�й㷺Ӧ�ã�X������A��Cԭ�Ӹ�����1��1��X�ĵ���ʽΪ ����д��Cu��ϡH2SO4��X��Ӧ�Ʊ�����ͭ�����ӷ���ʽ ��
��6��д��E��D������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽ ��
��1��1s22s22p63s23p1��2�֣�
��2��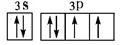 ��2�֣�
��2�֣�
��3��HClO4��2�֣�
��4������1�֣� �� ��1�֣� ����1�֣�
��5��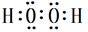 ��2�֣� Cu+2H++H2O2=Cu2++2H2O��2�֣�
��2�֣� Cu+2H++H2O2=Cu2++2H2O��2�֣�
��6��2Al+2NaOH+2H2O=2NaAlO2+3H2����2�֣�
�������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ���H��B��N��C��O��D��Na��E��Al��F��S��G��Cl����1��EԪ��ԭ�ӻ�̬�����Ų�ʽ1s22s22p63s23p1���𰸣�1s22s22p63s23p1����2���õ����Ų�ͼ��ʾFԪ�ؼ�SԪ��ԭ�ӵļ۵��ӹ���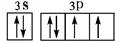 ���𰸣�
���𰸣� ����F ��GԪ�ض�Ӧ����ۺ�����H2SO4��HClO4�����Խ�ǿ�ķ���ʽΪHClO4���𰸣�HClO4���ȵ��Ӳ�ṹ��ͬ�����ӣ�ԭ������Խ��뾶ԽС��Na��<N3�D����һ������N>O��ͬ������ϵ��£��縺�Լ������縺�ԣ�O>S����;��������������H��O�γɵ�H2O2 �ĵ���ʽ��
����F ��GԪ�ض�Ӧ����ۺ�����H2SO4��HClO4�����Խ�ǿ�ķ���ʽΪHClO4���𰸣�HClO4���ȵ��Ӳ�ṹ��ͬ�����ӣ�ԭ������Խ��뾶ԽС��Na��<N3�D����һ������N>O��ͬ������ϵ��£��縺�Լ������縺�ԣ�O>S����;��������������H��O�γɵ�H2O2 �ĵ���ʽ��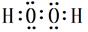 ��Cu��ϡH2SO4��H2O2��Ӧ�Ʊ�����ͭ�����ӷ���ʽ Cu+2H++H2O2=Cu2++2H2O����;
��Cu��ϡH2SO4��H2O2��Ӧ�Ʊ�����ͭ�����ӷ���ʽ Cu+2H++H2O2=Cu2++2H2O����; 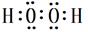 Cu+2H++H2O2=Cu2++2H2O����E��Al����NaOH��Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2��
Cu+2H++H2O2=Cu2++2H2O����E��Al����NaOH��Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2��
���㣺���ʵĽṹ

 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д������������(BN)n��һ�����ϳɲ��ϣ����ʽΪ(BN)�ݣ����и�Ӳ�ȡ����µ��ص㣬���������������մɲ��ϡ�ĥ�ϡ���ĥ�оߵĺò��ϡ�����ɰ��Na2B4O7���������ڸ��¸�ѹ�·�Ӧ���Ի�á����磺 Na2B4O7 + 2CO(NH2)2 �� 4(BN) + Na2O + 2CO2
��1���������������ʽ��ʾ��ɰ�Ļ�ѧʽ ������(BN)n�Ⱦ������и���Ӳ�Ⱥ������Ե�ԭ���ǣ� ��
��21��������Ӧʽ�о���4�ֲ�ͬ�������ӵ�ԭ�ӣ���������Ԫ�������ڱ��д��ڵ� ���ڣ��� �塣
��3�������ڱȽ�N��O�ǽ��������ǿ������ʵ�� ��
| A������������Ӧˮ��������� | B��H2O(g) ��NH3(g)�ȶ� |
| C��������H2��Ӧ�����׳̶� | D��NO�е�Ԫ�������ۣ���Ԫ���Ը��� |
��5������ͬ�������������ڵ�Ԫ�أ��������������NaOH��Һ��Ӧ�����ӷ���ʽ Ϊ�� ��
A��B��C��D��E���ֶ�����Ԫ�أ�ԭ���������������й���Ϣ���¡�
| Ԫ�� | �й���Ϣ |
| A | ����������Ӧ��ˮ���������⻯�ﷴӦ�������ӻ����� |
| B | �ؿ��к�������Ԫ�� |
| C | �����뱣����ú���� |
| D | ���ʼȿ������ᷴӦ���ֿ���NaOH��Һ��Ӧ |
| E | ԭ�������������ȴ�����������1�� |
��ش��������⣺
��1��A���⻯���ˮ��Һ��ʹ��̪��Һ��죬ԭ���ǣ��õ��뷽��ʽ��ʾ����ʵ������ȡ���⻯��Ļ�ѧ����ʽ�� ��
��2��A��B�����������Ϊ7��16����ԭ�ӷ��ӣ����л�������������ʵ��ŷ��йص��ǣ�����ţ���
������ ������ЧӦ �۳������ƻ� �ܹ⻯ѧ��Ⱦ
��3��C������������ˮ������E���ʷ�Ӧ��������������Һ�������ӷ���ʽ�� ��
��4��D��Ԫ�����ڱ��е�λ���ǡ���Ԫ��A��D���ij������������õ��Ե�ԣ���������ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
A��B��C��D��ԭ��������������Ķ���������Ԫ�أ�A��C��Ԫ�����ڱ��е����λ����ͼ��AԪ��������������ϵĵ�����֮��Ϊ3��BΪ�ؿ��к������Ľ���Ԫ�ء�
| A | |
| | C |
��1��Dԭ�ӽṹʾ��ͼΪ_____________��
��2����C�ĵͼ�̬�������ͨ�뵽D���ʵ�ˮ��Һ��ʹ֮��ɫ�������˼�________�ԣ�д���÷�Ӧ�����ӷ���ʽ_____________________��
��3��A������������Ӧ��ˮ�������ң��ֽ�����Cu���뵽100 mL 8.0 mol/L�ҵ�Ũ��Һ�У���ַ�Ӧ�����ռ���6.72L����״�������壬�������ijɷ���_________����ԭ��ʧ������Ϊ_________________��
��4��������������B���ʷֱ���뵽�������Ũ�ȵ������NaOH��Һ�У���ַ�Ӧ��������������Ϊ__________��������Ӧ�����õ���Һ��ϣ������ɰ�ɫ������������Ӧ�����ӷ���ʽΪ_____________________________________��B���ʱ��������Ĥ����NaOH��Һ��ȥ��д���÷�Ӧ�Ļ�ѧ����ʽ___________________________________��
X��Y��Z��T��W����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
| Ԫ�� | Ԫ�����ʻ�ԭ�ӽṹ |
| X | ԭ�ӵ������������Ǵ�����������2�� |
| Y | �����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
| Z | ��̬ԭ�����������Ų�ʽΪ��n+1��sn(n+1)pn2 |
| T | ��Zͬ���ڣ�Ԫ�������+7�� |
| W | ԭ������ΪY��TԪ��֮�ͣ�������к��и�Ԫ�� |
(1)Ԫ��X��һ��ͬλ�ؿɲⶨ�������������ͬλ�صķ�����______��WԪ�ػ�̬ԭ�ӵ����Ų�ʽ
Ϊ______________��
(2)Ԫ��Z��Ԫ��T��ȣ��ǽ����Խ�ǿ����_______(��Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ����___________________��
A.������Z�ĵ��ʺ�T�ĵ���״̬��ͬ
B. T���⻯���Z���⻯���ȶ�
C.һ��������Z��T�ĵ��ʶ���������������Һ��Ӧ
D. T�ĵ縺�Ա�Z��
(3)�����ܼ�XZ2�ķ����У����еĦҼ���м�������Ϊ______�������γɵľ�������Ϊ______�� Y�ij����⻯����Һ������Ҫԭ����________________________��
(4)Ԫ��X���Ԫ�ؿ����γ�һ�����ӻ�����û�����������Ӻ�CO��Ϊ�ȵ����壬�û�����ĵ���ʽΪ ������Ԫ��X���ӻ������� ��

