��Ŀ����
����˵����ȷ���ǣ��� ��
A����ӦA��g�� 2B��g������H��������Ӧ�Ļ��ΪEa kJ mol-1���淴Ӧ�Ļ��ΪEb kJ��mol-1�����H=��Ea��Eb��kJ��mol-1 2B��g������H��������Ӧ�Ļ��ΪEa kJ mol-1���淴Ӧ�Ļ��ΪEb kJ��mol-1�����H=��Ea��Eb��kJ��mol-1 |
| B����֪25��ʱ���й�����ĵ���ƽ�ⳣ����HCN Ka=4.9��10-10�� H2CO3 Ka1=4.3��10-7��Ka2=5.6��10-11����CO2ͨ��NaCN��Һ�з�Ӧ�Ļ�ѧ����ʽΪ��2NaCN+H2O+CO2=2HCN+Na2CO3 |
C����֪��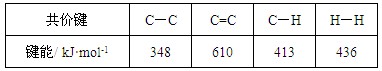 ��Ӧ 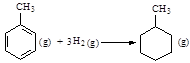 ���ʱ�Ϊ��H = ��384 kJ��mol-1 ���ʱ�Ϊ��H = ��384 kJ��mol-1 |
| D��һ��Ũ�ȵ�NaOH��Һ���¶�����PHֵ���� |
A
���ݵ���ƽ�ⳣ����֪��HCN������ǿ��HCO3���ģ�����B�ַ�ӦӦ������̼�����ƣ�����ȷ��C����ȷ����Ϊ���ֲ�����̼̼������̼̼˫�����¶����ߣ�ˮ�����ӻ�������������pH���ͣ�D����ȷ����ѡA��

��ϰ��ϵ�д�
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ
 2N2(g) +3H2O(g) ��H��0,��Ӧ����������________________��
2N2(g) +3H2O(g) ��H��0,��Ӧ����������________________�� N2O4(g) ��H��0���ֽ�һ�����Ļ������ͨ��һ�����ܱ������з�Ӧ,Ũ����ʱ��仯��ϵ��ͼ��ʾ����ͼ����������X��Y����ʾN2O4Ũ�ȱ仯����____��b��c��d����Ļ�ѧ��Ӧ���ʴ�С��ϵ��______��25minʱ�����߷���ͼ�б仯���ɲ�ȡ�Ĵ�ʩ��_________��
N2O4(g) ��H��0���ֽ�һ�����Ļ������ͨ��һ�����ܱ������з�Ӧ,Ũ����ʱ��仯��ϵ��ͼ��ʾ����ͼ����������X��Y����ʾN2O4Ũ�ȱ仯����____��b��c��d����Ļ�ѧ��Ӧ���ʴ�С��ϵ��______��25minʱ�����߷���ͼ�б仯���ɲ�ȡ�Ĵ�ʩ��_________��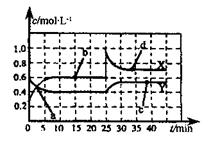
 N2O4(g) ��H <0��ƽ�ⳣ�� K��13.3������������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ���� c (NO2) =" 0.0300" mol��L��1��
N2O4(g) ��H <0��ƽ�ⳣ�� K��13.3������������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ���� c (NO2) =" 0.0300" mol��L��1��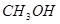 ��ԭ�ϡ���֪��
��ԭ�ϡ���֪��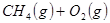
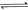
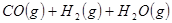
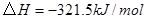
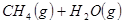
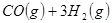
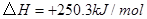
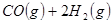
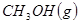
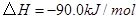
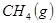 ��
��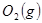 ��Ӧ����
��Ӧ����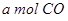 ��
��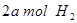 ���ڲ�ͬѹǿ�ºϳɼ״���
���ڲ�ͬѹǿ�ºϳɼ״���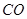 ��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��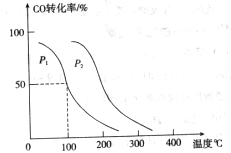
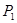
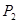 ���<������>����=����
���<������>����=����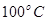 ��
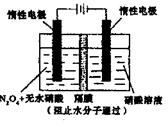
��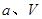 �Ĵ���ʽ��ʾ����
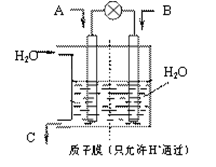
�Ĵ���ʽ��ʾ����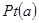 �缫ͨ���
�缫ͨ���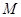 Ϊ ���缫��Ӧʽ�� ��
Ϊ ���缫��Ӧʽ�� ��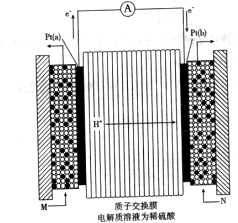
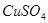 ��Һ�����õ�
��Һ�����õ�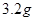 ͭʱ���μӷ�Ӧ������
ͭʱ���μӷ�Ӧ������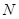 �����ӦΪ
�����ӦΪ 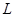 ����״������
����״������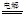 2OH?+H2��+Cl2��
2OH?+H2��+Cl2��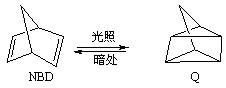 �����ø÷�Ӧ��������̫���ܣ���NBD�����ܱ�Q�����ܸ�
�����ø÷�Ӧ��������̫���ܣ���NBD�����ܱ�Q�����ܸ� 2NH3(g)+ 3/2O2(g)����H=+630kJ��mol-1
2NH3(g)+ 3/2O2(g)����H=+630kJ��mol-1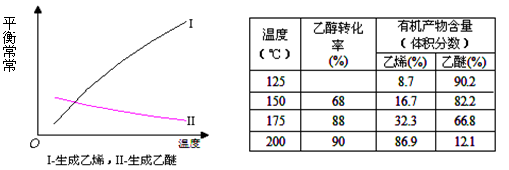
 2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ���������ƽ�� �ƶ�����������ҡ���������ʹ�ô�����������Ӧ�ġ�H________������� ����С�� ���ı䡱����
2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ���������ƽ�� �ƶ�����������ҡ���������ʹ�ô�����������Ӧ�ġ�H________������� ����С�� ���ı䡱���� ʽ�������ɸó��������ӷ���ʽΪ____________����֪25��ʱKsp[Mg��OH��2]=1��8��10-11,Ksp[Cu��OH��2]=2��2��10-20��
ʽ�������ɸó��������ӷ���ʽΪ____________����֪25��ʱKsp[Mg��OH��2]=1��8��10-11,Ksp[Cu��OH��2]=2��2��10-20��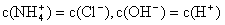
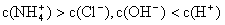
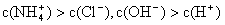
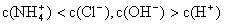
 NH3��H2O + H+����÷�Ӧ�����µ�ƽ�ⳣ��K= ����֪�������£�NH3��H2O�ĵ���ƽ�ⳣ��Kb=1��7��10��5 mol��L��1��
NH3��H2O + H+����÷�Ӧ�����µ�ƽ�ⳣ��K= ����֪�������£�NH3��H2O�ĵ���ƽ�ⳣ��Kb=1��7��10��5 mol��L��1��