��Ŀ����
�������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6����
��1����������ɵñ�ϩ��
��֪��C3H8(g) ==CH4(g)��HC
 CH(g)��H2(g) ��H1="156.6" kJ/mol
CH(g)��H2(g) ��H1="156.6" kJ/mol
CH3CH
 CH2(g)=CH4(g)��HC
CH2(g)=CH4(g)��HC
 CH(g ) ��H2="32.4" kJ/mol
CH(g ) ��H2="32.4" kJ/mol
����ͬ�����£���ӦC3H8(g) ==CH3CH CH2(g)��H2(g)�ġ�H= kJ/mol��
CH2(g)��H2(g)�ġ�H= kJ/mol��
��2���Ա���Ϊȼ����������ȼ�ϵ�أ���ص�����ͨ��O2��CO2������ͨ����飬�����������̼���Ρ�����ܷ�Ӧ����ʽΪ ���ŵ�ʱCO32�������ص� �����������������
��3��̼�⻯������ȫȼ������CO2��H2O�����³�ѹ�£������е�CO2����ˮ���ﵽƽ��ʱ����Һ��pH=5.60��c(H2CO3)=1.5��10-5 mol/L��������ˮ�ĵ��뼰H2CO3�ĵڶ������룬��H2CO3 HCO3����H����ƽ�ⳣ��K1= ������֪10-5.60=2.5��10-6��
HCO3����H����ƽ�ⳣ��K1= ������֪10-5.60=2.5��10-6��
��4�������£�0.1 mol/LNaHCO3��Һ��pH����8������Һ��c(H2CO3) c(CO32��)�����������=����������ԭ����
�������ӷ���ʽ�ͱ�Ҫ������˵������
��1����������ɵñ�ϩ��
��֪��C3H8(g) ==CH4(g)��HC

 CH(g)��H2(g) ��H1="156.6" kJ/mol
CH(g)��H2(g) ��H1="156.6" kJ/molCH3CH

 CH2(g)=CH4(g)��HC
CH2(g)=CH4(g)��HC
 CH(g ) ��H2="32.4" kJ/mol
CH(g ) ��H2="32.4" kJ/mol����ͬ�����£���ӦC3H8(g) ==CH3CH
 CH2(g)��H2(g)�ġ�H= kJ/mol��
CH2(g)��H2(g)�ġ�H= kJ/mol����2���Ա���Ϊȼ����������ȼ�ϵ�أ���ص�����ͨ��O2��CO2������ͨ����飬�����������̼���Ρ�����ܷ�Ӧ����ʽΪ ���ŵ�ʱCO32�������ص� �����������������
��3��̼�⻯������ȫȼ������CO2��H2O�����³�ѹ�£������е�CO2����ˮ���ﵽƽ��ʱ����Һ��pH=5.60��c(H2CO3)=1.5��10-5 mol/L��������ˮ�ĵ��뼰H2CO3�ĵڶ������룬��H2CO3
 HCO3����H����ƽ�ⳣ��K1= ������֪10-5.60=2.5��10-6��
HCO3����H����ƽ�ⳣ��K1= ������֪10-5.60=2.5��10-6����4�������£�0.1 mol/LNaHCO3��Һ��pH����8������Һ��c(H2CO3) c(CO32��)�����������=����������ԭ����
�������ӷ���ʽ�ͱ�Ҫ������˵������
��1��124.2 ��2�֣�
��2��C3H8��5O2=3CO2��4H2O ��2�֣� ����1�֣�
��3��4.2��10-7 mol/L��2�֣�
��4���� ��1�֣� HCO3����H2O CO32����H3O������HCO3��
CO32����H3O������HCO3�� CO32����H������
CO32����H������
HCO3����H2O H2CO3��OH����HCO3����ˮ��̶ȴ��ڵ���̶� ��3�֣�
H2CO3��OH����HCO3����ˮ��̶ȴ��ڵ���̶� ��3�֣�
��2��C3H8��5O2=3CO2��4H2O ��2�֣� ����1�֣�
��3��4.2��10-7 mol/L��2�֣�
��4���� ��1�֣� HCO3����H2O
 CO32����H3O������HCO3��
CO32����H3O������HCO3�� CO32����H������
CO32����H������HCO3����H2O
 H2CO3��OH����HCO3����ˮ��̶ȴ��ڵ���̶� ��3�֣�
H2CO3��OH����HCO3����ˮ��̶ȴ��ڵ���̶� ��3�֣������������1�����ݸ�˹���ɿɵã�?H=?H1-?H2="156.6" kJ?mol?1-32.4 kJ?mol?1=124.2kJ?mol?1��
��2��ȼ�ϵ�ص��ܷ�Ӧ����ʽ�ɸ���ȼ�շ�Ӧ�ķ���ʽ��д��C3H8��5O2=3CO2��4H2O��ԭ��طŵ�ʱ���������ƶ���
��3������pH�ɵ�c(H+)=10-5.6mol/L H2CO3 = HCO3? + H+
ƽ��Ũ�ȣ�mol?L?1�� 1.5��10-5 10-5.6 10-5.6
K=(10-5.6 mol?L?1��10-5.6 mol?L?1)��1.5��10-5 mol?L?1=4.2��10-7 mol?L?1��
��4��NaHCO3��Һ��������ƽ�⣬HCO3?�ĵ��룺HCO3��
 CO32����H����HCO3?��ˮ�⣺HCO3����H2O
CO32����H����HCO3?��ˮ�⣺HCO3����H2O H2CO3��OH������ΪNaHCO3��Һ��pH����8������HCO3?��ˮ��̶ȴ��ڵ���̶ȣ�c(H2CO3)>c(CO32��)��
H2CO3��OH������ΪNaHCO3��Һ��pH����8������HCO3?��ˮ��̶ȴ��ڵ���̶ȣ�c(H2CO3)>c(CO32��)��
��ϰ��ϵ�д�
�����Ŀ

 Si��s��+3HCl��g����ͬ�¶ȼ���ͬn��H2��/n��SiHCl3��ʱ����Ӧ��X��ƽ��ת���ʹ�ϵ��ͼ��
Si��s��+3HCl��g����ͬ�¶ȼ���ͬn��H2��/n��SiHCl3��ʱ����Ӧ��X��ƽ��ת���ʹ�ϵ��ͼ��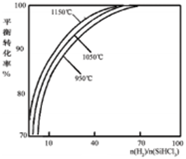

 2N2(g) +3H2O(g) ��H��0,��Ӧ����������________________��
2N2(g) +3H2O(g) ��H��0,��Ӧ����������________________�� N2O4(g) ��H��0���ֽ�һ�����Ļ������ͨ��һ�����ܱ������з�Ӧ,Ũ����ʱ��仯��ϵ��ͼ��ʾ����ͼ����������X��Y����ʾN2O4Ũ�ȱ仯����____��b��c��d����Ļ�ѧ��Ӧ���ʴ�С��ϵ��______��25minʱ�����߷���ͼ�б仯���ɲ�ȡ�Ĵ�ʩ��_________��
N2O4(g) ��H��0���ֽ�һ�����Ļ������ͨ��һ�����ܱ������з�Ӧ,Ũ����ʱ��仯��ϵ��ͼ��ʾ����ͼ����������X��Y����ʾN2O4Ũ�ȱ仯����____��b��c��d����Ļ�ѧ��Ӧ���ʴ�С��ϵ��______��25minʱ�����߷���ͼ�б仯���ɲ�ȡ�Ĵ�ʩ��_________��

 CH3OH��g�� ��H
CH3OH��g�� ��H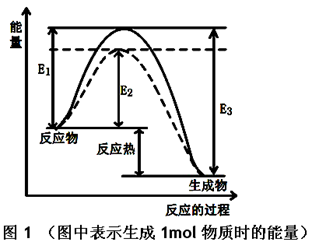

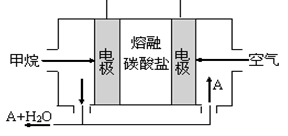
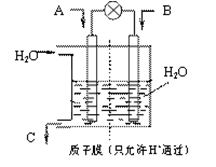
 N2O4(g) ��H <0��ƽ�ⳣ�� K��13.3������������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ���� c (NO2) =" 0.0300" mol��L��1��
N2O4(g) ��H <0��ƽ�ⳣ�� K��13.3������������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ���� c (NO2) =" 0.0300" mol��L��1��