��Ŀ����
����Ŀ������AgCl��Ag2CrO4���ܶȻ��ɵõ���ͼ��������Һ��Cl����������ʹ��AgNO3��Һ�ζ���Na2CrO4��ָʾ�������б����������
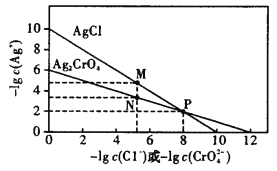
A.��ͼ��֪��Ag2CrO4���ܶȻ�С��AgCl���ܶȻ�
B.��ͼ��֪����Cl���ĵζ�ָʾ��ʱ��Na2CrO4��Ũ�Ȳ��ܹ���
C.��ͼ��֪��ֻ�е�c��Ag������10��2mol��L��1ʱ��![]() �ȳ���
�ȳ���
D.������AgNO3��Һ�ζ�������Һ��I����������Ϊ���ɵ�AgI������������I����ʹ�ζ��յ���ǰ
���𰸡�C
��������
A.��ͼ��֪��Ag2CrO4���ܶȻ�Ϊ10-12�� AgCl���ܶȻ�10-10������Ag2CrO4���ܶȻ�С��AgCl���ܶȻ�����A��ȷ��
B.��ͼ��֪��M��ʱ��Cl����Ũ��С��10-5 mol��L��1���ɿ���Cl��������ȫ��c(CrO-)���ܴ���N�㣬����Na2CrO4��Ũ�Ȳ��ܹ���B��ȷ��
C.��ͼ��֪������ܶȻ�����c(Ag��)=10-2mol��L-1ʱ��c(CrO-)=![]() =10-8 mol��L��1��c(C1-)=
=10-8 mol��L��1��c(C1-)=![]() =10-10 mol��L��1��c(CrO-)> c(C1-)�����Ե�c��Ag������10��2mol��L��1ʱ��AgC1�ȳ�������C����
=10-10 mol��L��1��c(CrO-)> c(C1-)�����Ե�c��Ag������10��2mol��L��1ʱ��AgC1�ȳ�������C����
D.������AgNO3��Һ�ζ�������Һ��I����������Ϊ���ɵ�AgI������������I����ʹ�ζ��յ���ǰ����D��ȷ��
��ѡC��

����Ŀ��������ѧ���������ϡ����ѧ������ȡ����Խ�ɾͣ�����Ϊ��ϡ�����Ԭ¡ƽ����ϡ��Ԫ�ذ����֡��ƺ���ϵԪ�ء���ش��������⣺
(1)д����̬����������(Sc2+)�ĺ�������Ų�ʽ____�����е���ռ�ݵĹ����Ϊ ____��
(2)�����������ⶨ��ϵԪ�غ�ʹ��ϵԪ�ط���ʱ������ʹ֮��ת���ɲ����Σ�Ȼ�����ն������������2LnCl3+3H2C2O4+nH2O=Ln2(C2O4)3nH2O+6HCl��
��H2C2O4��̼ԭ�ӵ��ӻ��������Ϊ____��1 mol H2C2O4�����к���������������Ŀ֮��Ϊ ___��
��H2O��VSEPRģ��Ϊ ___��д����H2O��Ϊ�ȵ������һ�������ӵĻ�ѧʽ_______��
��HCI��H2O�����γ�����ȶ���ˮ���������ξ��壬��HCl2H2O��HCl2H2O�к���H5O2+���ṹΪ![]() ���ڸ������У����ڵ���������___________
���ڸ������У����ڵ���������___________
a.��λ�� b.���Լ� c.�Ǽ��Լ� d.���Ӽ� e.������ f��� g.���»��� h.���� i.����
(3)�����г��˺˵����Ϊ21��25��Ԫ�ص���������ϼۣ�
Ԫ������ | �� | �� | �� | �� | �� |
Ԫ�ط��� | Sc | Ti | V | Cr | Mn |
�˵���� | 21 | 22 | 23 | 24 | 25 |
������� | +3 | +4 | +5 | +6 | +7 |
�Ա���������Ԫ��ԭ�ӵĺ�������Ų���Ԫ�ص���������ϼۣ��㷢�ֵĹ�����___________
(4)PrO2(��������)�ľ����ṹ��CaF2���ƣ�������Pr(��)ԭ��λ�����ĺͶ��㡣������������Prԭ����Oԭ��֮��ľ���Ϊa pm����þ�����ܶ�Ϊ_____gcm-3(��NA��ʾ�����ӵ�������ֵ�����ؼ�������)��