��Ŀ����
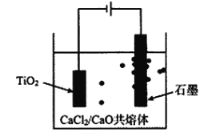
����Ŀ���ѹ���TiCl4�̳��к��д�����ScCl3��MgCl2��SiO2С���������ʣ�ij�о������������̳�����Sc2O3�����Ʊ��Ѱۣ�TiO2�����乤��������ͼ��ʾ��
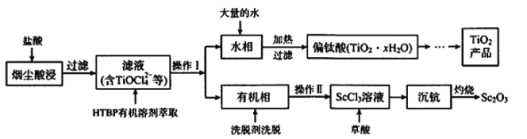
��1���ڿ��������ղ����ּ��ɵõ������֣�Sc2O3�����仯ѧ��Ӧ����ʽΪ_____��
��2����ˮ��������Ҫ������TiOCl42����H+��Cl����Mg2+��д�����������ˮ�����ȷ�����������ӷ�Ӧ����ʽ_____��
��3����������У��Թ�������������ó��ܽ������̳��⣬����е�������_______��
��4���ѵ�ұ���·��ǽ��ŵ�ⷨ����ͼ�����Ժ�����CaCl2��CaO��������Ϊ���ʣ����ʱ�����������ɵ�Ca��һ����ԭTiO2���ѡ�������ѧ��ѧ֪ʶ����Ԥ��CaCl2�����ð�����ǿ�����Լ�______��
���𰸡�3O2+2Sc2(C2O4)3![]() 2Sc2O3+12CO2 TiOCl42-+(1+x)H2O
2Sc2O3+12CO2 TiOCl42-+(1+x)H2O![]() TiO2��xH2O+2H++4Cl- Ϊ�˷�ֹTiOCl42-��Mg2+��Se3+ˮ�� ����CaO���۵㣬��Լ����
TiO2��xH2O+2H++4Cl- Ϊ�˷�ֹTiOCl42-��Mg2+��Se3+ˮ�� ����CaO���۵㣬��Լ����
��������
(1)�ڿ��������ղ����ּ��ɵõ�������(Sc2O3)��ͬʱ��������ӱ��������ɶ�����̼����Ӧ�Ļ�ѧ��Ӧ����ʽΪ3O2+2Sc2(C2O4)3![]() 2Sc2O3+12CO2��
2Sc2O3+12CO2��
(2)��������ͼ���ѹ���TiCl4�̳��к��д�����ScCl3��MgCl2��SiO2С���������ʣ�������˳�ȥ�˶������裬�����л��ܼ���ȡ����ˮ��������Ҫ������TiOCl42-��H+��Cl-��Mg2+����ˮ���м��������ˮ�����ȴٽ�TiOCl42-ˮ������TiO2��xH2O����Ӧ�����ӷ���ʽΪTiOCl42-+(1+x)H2O![]() TiO2��xH2O+2H++4Cl-��
TiO2��xH2O+2H++4Cl-��
(3) ��������У��Թ�������������ó��ܽ������̳��⣬����е�������Ϊ�˷�ֹTiOCl42-��Mg2+��Se3+ˮ�⣻
(4)�����Ϊ�����Ȼ��Ƶ����ڵ������ƣ������Ȼ��ƿ�����ǿ����ʵĵ����ԣ�ͬʱ���Խ���CaO���۵㣬��Լ������

����Ŀ��ij��ѧС��ͬѧ������װ�ú��Լ�����ʵ�飬̽��O2��KI��Һ������Ӧ��������
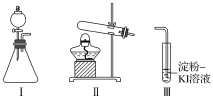
��ѡ�Լ���30%H2O2��Һ��0.1mol/L H2SO4��Һ��MnO2���塢KMnO4����
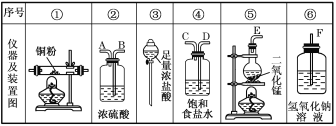
��1��С��ͬѧ��Ƽס��ҡ�������ʵ�飬��¼����
���� | ���� | |
�� | ��I����ƿ�м���___����I��____�м���30%H2O2��Һ������I������ | I�в�����ɫ���岢�����������������������ð������ҺѸ�ٱ��� |
�� | ����м���KMnO4���壬���Ӣ�ȼ�ƾ��� | ����������ð������Һ������ |
�� | ����м���KMnO4���壬���м�������0.1mol/LH2SO4��Һ�����Ӣ�ȼ�ƾ��� | ����������ð������Һ���� |
��2����ʵ����O2��KI��Һ��Ӧ�����ӷ���ʽ��____��
��3���Ա��ҡ���ʵ���֪��O2��KI��Һ������Ӧ������������___��Ϊ��һ��̽���������Է�Ӧ���ʵ�Ӱ�죬�ɲ�ȡ��ʵ���ʩ��___��
��4���ɼס��ҡ�����ʵ���Ʋ⣬��ʵ�������I�еİ���ʹ��Һ������ѧ����I�в���������ֱ��ͨ������_____(����ĸ)��Һ��֤���˰����к���H2O2��
A������KMnO4 B��FeC12 C��Na2S D��Ʒ��
����Ŀ��(1)��֪Ksp[Cu(OH)2]��2.2��10��20��Ksp[Fe(OH)3]��2.6��10��39�������£�ij����CuCl2��Һ�к���������FeCl3��Ϊ�˵õ�������CuCl2��2H2O���壬Ӧ����___________(��������Ļ�ѧʽ)��������Һ��pH��4��ʹ��Һ�е�Fe3��ת��ΪFe(OH)3��������ʱ��Һ�е�c(Fe3��)��________�����˺�������Һ����������Ũ���ᾧ���ɵõ�CuCl2��2H2O���塣
(2)ij̼�ظֹ�¯��ˮ������Ҫ�ɷ���̼��ơ�����ơ�������þ�����⡢��������ȡ�ˮ���輰ʱ��ϴ��ȥ����ϴ�������£�
��.����NaOH��Na2CO3���Һ�����ȣ�������Сʱ��
��.�ų�ϴ�ӷ�Һ����ˮ��ϴ��¯������ϡ���������NaF��Һ�����ݣ�
��.��ϴ��Һ�м���Na2SO3��Һ��
��.��ϴ��꣬��NaNO2��Һ�ۻ���¯��
����ϡ�����ܽ�̼��Ƶ����ӷ���ʽ��_____________________________��
����֪��25 ��ʱ�й����ʵ��ܶȻ�
���� | CaCO3 | CaSO4 | Mg(OH)2 | MgCO3 |
Ksp | 2.8��10��9 | 9.1��10��6 | 1.8��10��11 | 6.8��10��6 |
�������ݣ���ϻ�ѧƽ��ԭ��������ϴCaSO4�Ĺ���________________�������ܽ�ƽ�����ʽ�ͱ�Ҫ��������������˵�������ڲ������ݹ����л��ᷢ����ӦMgCO3(s)��2OH��(aq)![]() Mg(OH)2(s)��CO32-(aq)���÷�Ӧ��ƽ�ⳣ��K��________(������λ��Ч����)��
Mg(OH)2(s)��CO32-(aq)���÷�Ӧ��ƽ�ⳣ��K��________(������λ��Ч����)��
�۲�����У�����Na2SO3��Һ��Ŀ����_______________________________��
����Ŀ������һ������Һ��ֻ���ܺ����±��е�����������(���ڵ����Ӿ�����)��
������ | K����NH4+��H����Ba2�� |
������ | Cl����CO32-��SO42-��OH�� |
��ȡ��������������Һ��100 mL���ֱ��������ʵ�飺
�ٵ�һ�ݼ���AgNO3��Һ�г���������
�ڵڶ��ݼ�������NaOH��Һ���Ⱥ��ռ�������0.04 mol��
�۵����ݼ�������BaCl2��Һ�ø������6.27 g������������ϴ�ӣ������������Ϊ2.33 g��
��ش��������⣺
��1�����ݵڢڸ�ʵ�����ȷ�����ڵ�������_______��
��2������1����ȷ�����ڵ��������⣬��һ�����ڵ�������__________�����ܴ��ڵ�������______��
��3���ڢ۸�ʵ���г������ٵ�ԭ����(�����ӷ���ʽ��ʾ)______________��