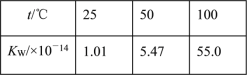��Ŀ����
����Ŀ��(1)��֪Ksp[Cu(OH)2]��2.2��10��20��Ksp[Fe(OH)3]��2.6��10��39�������£�ij����CuCl2��Һ�к���������FeCl3��Ϊ�˵õ�������CuCl2��2H2O���壬Ӧ����___________(��������Ļ�ѧʽ)��������Һ��pH��4��ʹ��Һ�е�Fe3��ת��ΪFe(OH)3��������ʱ��Һ�е�c(Fe3��)��________�����˺�������Һ����������Ũ���ᾧ���ɵõ�CuCl2��2H2O���塣
(2)ij̼�ظֹ�¯��ˮ������Ҫ�ɷ���̼��ơ�����ơ�������þ�����⡢��������ȡ�ˮ���輰ʱ��ϴ��ȥ����ϴ�������£�
��.����NaOH��Na2CO3���Һ�����ȣ�������Сʱ��
��.�ų�ϴ�ӷ�Һ����ˮ��ϴ��¯������ϡ���������NaF��Һ�����ݣ�
��.��ϴ��Һ�м���Na2SO3��Һ��
��.��ϴ��꣬��NaNO2��Һ�ۻ���¯��
����ϡ�����ܽ�̼��Ƶ����ӷ���ʽ��_____________________________��
����֪��25 ��ʱ�й����ʵ��ܶȻ�
���� | CaCO3 | CaSO4 | Mg(OH)2 | MgCO3 |
Ksp | 2.8��10��9 | 9.1��10��6 | 1.8��10��11 | 6.8��10��6 |
�������ݣ���ϻ�ѧƽ��ԭ��������ϴCaSO4�Ĺ���________________�������ܽ�ƽ�����ʽ�ͱ�Ҫ��������������˵�������ڲ������ݹ����л��ᷢ����ӦMgCO3(s)��2OH��(aq)![]() Mg(OH)2(s)��CO32-(aq)���÷�Ӧ��ƽ�ⳣ��K��________(������λ��Ч����)��
Mg(OH)2(s)��CO32-(aq)���÷�Ӧ��ƽ�ⳣ��K��________(������λ��Ч����)��
�۲�����У�����Na2SO3��Һ��Ŀ����_______________________________��
���𰸡�CuO 2.6��10��9 mol��L��1 CaCO3��2H��===Ca2����H2O��CO2�� CaSO4��ˮ�д����ܽ�ƽ��CaSO4(s) ![]() Ca2��(aq)��SO42-(aq)����Na2CO3��Һ���ݣ�Ca2����CO32-��ϳɸ����ܵ�CaCO3��ʹ����ƽ�����ƣ�CaSO4ת����CaCO3��Ȼ����ϡ�����ȥ 3.8��105 ��Fe3����ԭ��Fe2������ֹFe3����ʴ��¯
Ca2��(aq)��SO42-(aq)����Na2CO3��Һ���ݣ�Ca2����CO32-��ϳɸ����ܵ�CaCO3��ʹ����ƽ�����ƣ�CaSO4ת����CaCO3��Ȼ����ϡ�����ȥ 3.8��105 ��Fe3����ԭ��Fe2������ֹFe3����ʴ��¯
��������
��1�����ݳ���ԭ����������������������ܶȻ��������㣻
��2���������̼��Ʒ�Ӧ�����Ȼ��ơ�������̼��ˮ��
�ڸ��ݱ����������Լ��ܽ�ƽ������жϣ�
�۸����������ƾ��л�ԭ�Է�����
��1��Ϊ�˵õ�������CuCl2��2H2O������Ҫ��ȥ�Ȼ�������������ʺ��Ȼ�����Ӧ�������������Ҳ��������µ����ʣ��Ҽ������ʺ�Ӧ��ת��Ϊ�Ȼ�ͭ������Ӧ�ü��뺬ͭԪ�ص����ʣ���˸���������CuO��������Һ��pH��4��ʹ��Һ�е�Fe3��ת��ΪFe(OH)3��������ʱ��Һ��������Ũ����10��4mol/L��������������Ũ����10��10mol/L������������������ܶȻ�������֪��Һ��c(Fe3��)��![]() mol/L��2.6��10��9 mol��L��1��
mol/L��2.6��10��9 mol��L��1��
��2������ϡ�����ܽ�̼��Ƶ����ӷ���ʽ��CaCO3��2H����Ca2����H2O��CO2����
�ڸ��ݱ������ݿ�֪̼��Ʊ�����Ƹ����ܣ�CaSO4��ˮ�д����ܽ�ƽ��CaSO4(s)![]() Ca2��(aq)��SO42-(aq)����Na2CO3��Һ���ݣ�Ca2����CO32-��ϳɸ����ܵ�CaCO3��ʹ����ƽ�����ƣ�ʹCaSO4ת����CaCO3�������ϡ�����ȥ�����ݷ�ӦMgCO3(s)��2OH��(aq)
Ca2��(aq)��SO42-(aq)����Na2CO3��Һ���ݣ�Ca2����CO32-��ϳɸ����ܵ�CaCO3��ʹ����ƽ�����ƣ�ʹCaSO4ת����CaCO3�������ϡ�����ȥ�����ݷ�ӦMgCO3(s)��2OH��(aq)![]() Mg(OH)2(s)��CO32-(aq)��֪�÷�Ӧ��ƽ�ⳣ��K��
Mg(OH)2(s)��CO32-(aq)��֪�÷�Ӧ��ƽ�ⳣ��K��![]() ��3.8��105��
��3.8��105��
�۲�����м���Na2SO3��Һ���������ӷ���������ԭ��Ӧ����Ŀ����Ϊ�������ӻ�ԭΪ�������ӣ���ֹ�����ӱ���ʴ��

����Ŀ�����и���ʵ�������ʵ���������ó��Ľ�������ȷ����
ѡ�� | ʵ����� | ʵ������ | ���� |
A | ��FeCl2��Ʒ��������μ�KSCN��Һ | ��Һ��ɺ�ɫ | ԭFeCl2��Ʒ�ѱ��� |
B | ��Na2SO3��Ʒ����ˮ���μ���ϡ�����ữ��Ba(NO3)2��Һ | ������ɫ���� | ԭNa2SO3��Ʒ�ѱ��� |
C | �����KI��Һ�е���3��ϡ���ᣬ�ټ���10%��H2O2��Һ | ����ϡ����δ����Һ����������H2O2��Һ����Һ�������� | ���������£�H2O2������ǿ��I2 |
D | ��5mL0.5mol/LNaHCO3��Һ�е���2mL1mol/LBaCl2��Һ | ������ɫ������������ɫ�������� | ��Ӧ�Ļ�ѧ����ʽΪ2NaHCO3+BaCl2=BaCO3��+ 2NaCl+CO2��+H2O |
A. AB. BC. CD. D
����Ŀ�������Ƿdz����õIJ��ϣ����������ֽ�����������ʡ�
���� | �۵㣨�棩 | �ܶȣ�g/cm3�� | ���ǿ�� ��1��ʾ������ | ���Ӳ�� ��1��ʾ������ | ÿ�ּ۸� ��Ԫ�� |
�� | 660 | 2.7 | 11 | 2.8 | 11400 |
ͭ | 1085 | 8.9 | 33 | 3.0 | 38000 |
�� | 1538 | 7.9 | 20 | 4.5 | 4000 |
�� | 1668 | 4.5 | 40 | 6.0 | 160000 |
���������գ�
(1)����������������������_________�ԣ�����������������������_________�ԡ�
(2)��ҵ�ϳ���CuΪԭ���Ʊ�CuSO4������ɫ��ѧ��Ҫ��Ӿ��á������ͻ�������ƿ��еĻ�ѧ��Ӧ������Ӧ�������ٶԻ����ĸ����á����з�Ӧ���ϡ���ɫ��ѧ��������_______��
A��Cu + 2H2SO4(Ũ) ![]() CuSO4 + SO2�� + 2H2O
CuSO4 + SO2�� + 2H2O
B��2Cu + O2![]() 2CuO��CuO + H2SO4��CuSO4 +H2O
2CuO��CuO + H2SO4��CuSO4 +H2O
(3)����������Ũ������ʹ��___________�����Ũ����������۳����䡣
(4)��������ɻ��IJ���֮һ�����ݱ������ݣ�����Ϊ������������ɻ���______________
(5)������Ҫ�������ĺϽ�����������������������졣��֪���ڳ�ʪ�Ŀ����л�Ѹ�ٸ�ʴ���������ᡣ���������ʴ��ԭ��_______________________________________
(6)���ݱ������ݣ�����������ȡ����������������һ���ŵ��һ��ȱ�㡣
�ŵ㣺_____________________________________________________________________
ȱ�㣺_____________________________________________________________________