��Ŀ����
15�� �������ַ����Ʊ�����������
�������ַ����Ʊ�������������2.7gAl$\stackrel{100mLϡ����}{��}$X��Һ$\stackrel{����������Һ}{��}$��������
��2.7gAl$\stackrel{100mL����������Һ}{��}$Y��Һ$\stackrel{ϡ����}{��}$��������
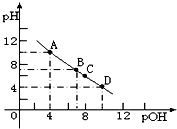
��֪���õ�ϡ����������������Һ��Ϊ3mol/L��ͼ����X��Һ��Y��Һ�зֱ��������������Һ��ϡ����ʱ���������������������Һ���֮��Ĺ�ϵ������Ϻ�����仯���Բ��ƣ�����˵����ȷ���ǣ�������
| A�� | ��a���߱�ʾ������Y��Һ�м���ϡ���� | |
| B�� | ������ȷ��������ɸ�������� | |
| C�� | ��M��ʱ����������������Һ����������������ͬ | |
| D�� | M���Ժ�a��b�������߽��غ�Ϊһ�� |
���� ����I�ᷢ����Ӧ��2Al+6HCl=2AlCl3+3H2����AlCl3+3NaOH=Al��OH��3��+3NaCl��
������ᷢ����Ӧ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����NaAlO2+HCl+H2=Al��OH��3��+NaCl��
�������ʵ���=$\frac{2.7g}{27g/mol}$=0.1mol��n��HCl��=n��NaOH��=3mol•L-1��0.1L=0.3mol�����ݷ�Ӧ����ʽ����֪����I��Al��HClǡ�÷�Ӧ����������NaOH��ʣ�࣮
A������I���ٵμ�NaOH���������ɳ��������������ټ���HCl�����к�ʣ���NaOH���ٷ�Ӧ������������������
B�����ݷ���ʽ������м��㣻
C��M�����վ�ΪNaCl��Һ����Һ������䣬����NaCl��ȣ�
D��������Ӧ��Al��OH��3+3HCl=AlCl3+3H2O��Al��OH��3+NaOH�TNaAlO2+2H2O�����������ܽ���������������������Һ�������ȣ�
��� �⣺����I�ᷢ����Ӧ��2Al+6HCl=2AlCl3+3H2����AlCl3+3NaOH=Al��OH��3��+3NaCl��
������ᷢ����Ӧ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����NaAlO2+HCl+H2=Al��OH��3��+NaCl��
�������ʵ���=$\frac{2.7g}{27g/mol}$=0.1mol��n��HCl��=n��NaOH��=3mol•L-1��0.1L=0.3mol�����ݷ�Ӧ����ʽ����֪����I��Al��HClǡ�÷�Ӧ����������NaOH��ʣ�࣮
A������I���ٵμ�NaOH���������ɳ��������������ټ���HCl�����к�ʣ���NaOH���ٷ�Ӧ������������������������a���߱�ʾ������X��Һ�м���NaOH��Һ����A����
B��Al��ȫ��Ӧ�����ݷ���ʽ��֪���������������ȣ���B����
C��M�����վ�ΪNaCl��Һ����Һ������䣬����NaCl��ȣ�����M��ʱ����������������Һ����������������ͬ����C��ȷ��
D��������Ӧ��Al��OH��3+3HCl=AlCl3+3H2O��Al��OH��3+NaOH�TNaAlO2+2H2O�����������ܽ���������������������Һ�������ȣ�M���Ժ�a��b�������߲����غ�Ϊһ������D����
��ѡC��
���� ���������������Ʊ�����Ϊ���壬���黯ѧ����ʽ���㡢��ѧ��Ӧͼ������ȣ���ȷ�����ķ�Ӧ�ǽ���ؼ����Ѷ��еȣ�

 ijУ����ʵ��С��ͬѧ�������ͼװ����ȡ���ռ�һƿ���壬�������С��ͬѧ����±���
ijУ����ʵ��С��ͬѧ�������ͼװ����ȡ���ռ�һƿ���壬�������С��ͬѧ����±���| ��ȡ������ | ҩ Ʒ | ��ѧ����ʽ |
| O2 | H2O2��MnO2 | 2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2�� |
| H2 | �Ҵ���Na | 2CH2CH2OH+2Na=2CH3CH2ONa+H2�� |
| A�� | �¶�һ��ʱ������Һ��c��Ba2+����c��SO42-��=1.07��10-10ʱ������ҺΪBaSO4�ı�����Һ | |
| B�� | ��ΪKsp��BaCO3����Ksp��BaSO4������������BaSO4ת��ΪBaCO3 | |
| C�� | 25��ʱ����δ�ܽ���BaCO3�ı�����Һ�е�������Na2SO4��Һ����BaSO4������������ʱ��Һ��c��CO32-����c��SO42-��=24.11 | |
| D�� | �ڱ���BaCO3��Һ�м�������Na2CO3���壬��ʹc��Ba2+����С��BaCO3���ܶȻ����� |
| A�� | Fe2O3�ʺ���ɫ����������ɫ���� | |
| B�� | Ũ�����ܸ�ʴ�������������κν�������ʢװŨ���� | |
| C�� | �����ж���������һ���ʿɽ�������ˮ��ɱ������ | |
| D�� | þ�������Ƚ�������������װ�β��� |
| A�� | �ö��Ե缫���CuCl2��Һ����������16gͭʱ����·��ͨ���ĵ�����ΪNA | |
| B�� | ��״���£�2.24L��ȩ��ȫȼ������CO2������ԼΪ0.2NA | |
| C�� | ��״���£�22.4L�����к���NA����ԭ�� | |
| D�� | 1mol������������ԼΪ8NA |
| A�� | �������в�����̼̼˫�������Բ��ܷ����ӳɷ�Ӧ | |
| B�� | �Ҵ��ܷ���������Ӧ���������ܷ���������Ӧ | |
| C�� | ������Ҵ������������뱽��������ˮ���Ʊ�������Ҵ�������ȡ����Ӧ | |
| D�� | �ױ��������ڹ����·�Ӧ��Ҫ����2��4-���ȼױ� |
| A�� | 1mol${\;}_{8}^{16}$OD- �к��е�����������������Ϊ9NA | |
| B�� | 3.6 gʯī��C60�Ļ�����У����е�̼ԭ����Ϊ0.3NA | |
| C�� | ��Ӧ3H2��g��+N2��g��?2NH3��g����H=-92kJ/mol�ų�����9.2kJʱ��ת�Ƶ���0.6NA | |
| D�� | ��״���£�4.48L���麬�еķ�����Ϊ0.2NA |
| A�� | ��������ϴ��¯�е�ˮ�� | |
| B�� | ��Һ�ǵ����Եģ������Ǵ���� | |
| C�� | ���ö����ЧӦ����������Һ�뽺�� | |
| D�� | �ռ�����ᡢ���Ȼ�̼��Ϊ����� |
 ����ʱ������������ˮ�γ�һŨ��Ϊ0.1mol/L��pHΪ10�İ�ˮ��Һ100mL��
����ʱ������������ˮ�γ�һŨ��Ϊ0.1mol/L��pHΪ10�İ�ˮ��Һ100mL��