��Ŀ����
5��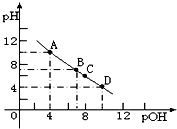 ����ʱ������������ˮ�γ�һŨ��Ϊ0.1mol/L��pHΪ10�İ�ˮ��Һ100mL��
����ʱ������������ˮ�γ�һŨ��Ϊ0.1mol/L��pHΪ10�İ�ˮ��Һ100mL����1���ð�ˮ��Һ�к��е�����NH3•H2O��OH-��H2O��NH3��NH4+��H+��
��2���ð�ˮ��Һ��ˮ�������c��OH-��Ϊ10-10mol/L��
��3����������ˮ���ð�ˮϡ��10����pH=a����a��ȡֵ��ΧΪ9��a��10��$\frac{{c��N{H_3}•{H_2}O��•c��O{H^-}��}}{{c��{H^+}��}}$��ֵ��С������������䡱���١�����
��4������ð�ˮ����μ����Ũ�ȵ����ᣬ��Һ��pH��pOH��pOH=-lg[OH-]���ı仯��ϵ��ͼ��ʾ��A����ʾ��Һ�ĵ�������С�ڣ�����ڡ��������ڡ���С�ڡ���D�㣬B��������������С�ڣ�����ڡ��������ڡ���С�ڡ���100mL��C����Һ�����Ե�ԭ���ǣ������ӷ���ʽ��ʾ��NH4++H2O?NH3•H2O+H+��
���� ��1����ˮ�д���ˮ�ĵ���ƽ���һˮ�ϰ��ĵ���ƽ�⣬�ݴ��жϰ�ˮ�к��е����ӣ�
��2�������pH=10����Һ�е�������Ũ�ȣ���ˮ�е���������ˮ����ģ��ݴ˼����ˮ���������������Ũ�ȣ�
��3��һˮ�ϰ�Ϊ������ʣ�ϡ�����е���������������ӵ����ʵ���������ϡ�ͺ���Һ��pH��9������ϡ������һˮ�ϰ�������������Ũ�ȼ�С��������Ũ����������жϣ�
��4��A��Ϊ��ˮ��Һ��D��Ϊ�Ȼ�狀��Ȼ���Ļ��Һ��A����Һ������Ũ��С��D��B��pH=pOH����ҺΪ���ԣ���������������Ϊ100mLʱ��ǡ�÷�Ӧ�����Ȼ�泥���ҺΪ���ԣ���Ϊ���ԣ��������������С��100ml��C��ʱ����Ϊ�Ȼ�泥�笠����Ӳ���ˮ�⣬��Һ��ʾ���ԣ�
��� �⣺��1����ˮ�д���ˮ�ĵ���ƽ���һˮ�ϰ��ĵ���ƽ�⣬��ˮ�к��е����У�NH3•H2O��OH-��H2O��NH3��NH4+��H+��
�ʴ�Ϊ��NH3��NH4+��H+��
��2����ˮ�����������������������ˮ�ĵ��룬��ˮ�е���������ˮ����ģ�pH=10����Һ�У�������Ũ��Ϊ10-10mol/L��ˮ����������Ӻ�����������Ũ����ȣ���ˮ���������������Ũ��Ϊ10-10 mol/L��
�ʴ�Ϊ��10-10mol/L��
��3����ˮΪ�����������ˮ��pH=10��ˮϡ��10����pH=a������ϡ�ͺ���Һ�����������ӵ����ʵ���������ϡ�ͺ���Һ��pH��9������a�ķ�ΧΪ��9��a��10��ϡ�����У���Һ��һˮ�ϰ�������������Ũ�ȶ���С����ˮ�����ӻ����䣬����Һ��������Ũ���������Ըñ�ֵ���٣�
�ʴ�Ϊ��9��a��10�����٣�
��4��A��Ϊ��ˮ��Һ������������Ũ��Ϊ10-4mol/L��D��Ϊ�Ȼ�狀��Ȼ���Ļ��Һ��������Ũ��Ϊ��10-4mol/L��A����Һ������Ũ��С��D����D��ʱ��Һ�����Խ�ǿ��
B��pH=pOH����ҺΪ���ԣ���������������Ϊ100mLʱ��ǡ�÷�Ӧ�����Ȼ�泥���ҺΪ���ԣ���������ҺΪ���ԣ��������������С��100mL��
C��ʱӦ��Ϊ��ˮ������ǡ�÷�Ӧ����ʱ����Ϊ�Ȼ�泥�笠����Ӳ���ˮ�⣺NH4++H2O?NH3•H2O+H+����Һ��ʾ���ԣ�
�ʴ�Ϊ��С�ڣ�С�ڣ�NH4++H2O?NH3•H2O+H+��
���� ���⿼��������ϵĶ����жϼ���ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ�ע��������Һ���������ҺpH��ϵ�����㷽������ȷ��Ӧ���������Ϊ���ؼ���������ؿ���ѧ���ķ������������������Ӧ�û���֪ʶ��������

 53���ò�ϵ�д�
53���ò�ϵ�д� �������ַ����Ʊ�����������
�������ַ����Ʊ�������������2.7gAl$\stackrel{100mLϡ����}{��}$X��Һ$\stackrel{����������Һ}{��}$��������
��2.7gAl$\stackrel{100mL����������Һ}{��}$Y��Һ$\stackrel{ϡ����}{��}$��������
��֪���õ�ϡ����������������Һ��Ϊ3mol/L��ͼ����X��Һ��Y��Һ�зֱ��������������Һ��ϡ����ʱ���������������������Һ���֮��Ĺ�ϵ������Ϻ�����仯���Բ��ƣ�����˵����ȷ���ǣ�������
| A�� | ��a���߱�ʾ������Y��Һ�м���ϡ���� | |
| B�� | ������ȷ��������ɸ�������� | |
| C�� | ��M��ʱ����������������Һ����������������ͬ | |
| D�� | M���Ժ�a��b�������߽��غ�Ϊһ�� |
| ��� | ʵ��Ҫ�� | �� | ��ѡ��Ļ�ѧ�Լ������� |
| a | ���������Ƿ�������� | A�����Ƶ�������ͭ����Һ | |
| b | ����ֲ�������Ƿ���̼̼˫�� | B����ɫʯ����Һ | |
| c | ������Һ���Ƿ��������� | C�����뱥��Na2CO3��Һ����Һ | |
| d | ��ȥ���������е��������� | D����ˮ |

��д���������������Ľ����Ʒ�����Ӧ�Ļ�ѧ����ʽ
 +4Na��
+4Na�� +2H2����
+2H2���� | A�� | PbO2�õ��ӣ������� | |
| B�� | Ǧ���ع���������ÿͨ��2mol���ӣ�������������207g | |
| C�� | ��������PbO2�����·����Pb | |
| D�� | ��طŵ�ʱ����Һ������ǿ |
| A�� | ���� | B�� | �������� | C�� | �� | D�� | ���� |
 ��ͭ��ĩ����ɫ��Ϊ��ɫ�����ɵ������F�����ݳ����������ȼ���ȼ�գ�
��ͭ��ĩ����ɫ��Ϊ��ɫ�����ɵ������F�����ݳ����������ȼ���ȼ�գ� ��A��һ��ͬ���칹��B�����ܺ�Na2CO3��Һ��Ӧ����CO2����B�Ľṹ��ʽΪCH3COOH��A����һ��ͬ���칹��C��������������ˮ��õ�D��E����D��E����Է���������ͬ��д��C������ˮ�ⷴӦ�Ļ�ѧ����ʽ��
��A��һ��ͬ���칹��B�����ܺ�Na2CO3��Һ��Ӧ����CO2����B�Ľṹ��ʽΪCH3COOH��A����һ��ͬ���칹��C��������������ˮ��õ�D��E����D��E����Է���������ͬ��д��C������ˮ�ⷴӦ�Ļ�ѧ����ʽ�� ��
��