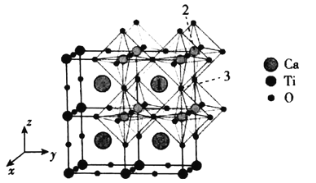
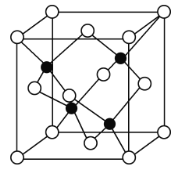
��Ŀ����
����Ŀ���������ڻ�������������Ҫ���á�ij��ȤС����50%����������������������������������������Ʊ������ᣬ�䷴Ӧԭ��Ϊ![]()
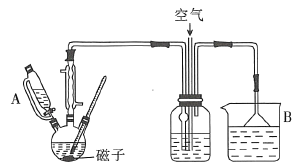
![]() ��ʵ��װ����ͼ��ʾ��
��ʵ��װ����ͼ��ʾ��
ʵ�鲽�裺��װ�л��������ܡ��¶ȼƵ�![]() ������ƿ�У�����50%
������ƿ�У�����50%![]() ��Һ����
��Һ����![]()
![]() ������������泥�
������������泥�![]() ���������μ�5~6�λ��������к���ɫ�������������������ʣ��Ļ������μ���ϣ�����Ϊ
���������μ�5~6�λ��������к���ɫ�������������������ʣ��Ļ������μ���ϣ�����Ϊ![]() ��Լ
��Լ![]() �������¶�Ϊ80~90��ʱ����Ӧ������ɫ�����ݳ�������ӦҺ����
�������¶�Ϊ80~90��ʱ����Ӧ������ɫ�����ݳ�������ӦҺ����![]() ���ձ��У���ȴ�����������ᡣ��ѹ���ˣ���
���ձ��У���ȴ�����������ᡣ��ѹ���ˣ���![]() ��ˮϴ�ӣ������õ��ֲ�Ʒ
��ˮϴ�ӣ������õ��ֲ�Ʒ![]() ��
��
�ش��������⣺
��1������A������Ϊ________������B����ʢװ���Լ�Ϊ________��
��2��ʵ������У������˵ļ��ȷ���Ϊ________���ü��ȷ������ŵ���________��
��3����ʵ���г�������淋�����Ӧѡ��________������������ƽ������������ƽ������
��4����ѹ���˺�ľ�������ˮϴ�ӣ�����ϴ�ӵIJ������̣�________��
��5��Ϊ�ⶨ�ֲ�Ʒ�м�����ĺ��������õ��Ĵֲ�Ʒ�����Һ������![]() ��
��![]() ����Һ���еζ������в�������ʹʵ������
����Һ���еζ������в�������ʹʵ������![]() ����Һ�����ƫ�����________�����ţ���
����Һ�����ƫ�����________�����ţ���
A.ʹ�ü�����ָʾ��
B.�ζ�ǰ���Ӷ������ζ������Ӷ���
C.ʵ���õļ�ʽ�ζ��ܡ���ƿˮϴ���δ��ϴ
��6������������صõ�![]() ���Ƽ����ᣬ����IJ���Ϊ________��������λ��Ч���֣���
���Ƽ����ᣬ����IJ���Ϊ________��������λ��Ч���֣���
���𰸡���ѹ��Һ©�����Һ©�� NaOH��Һ ˮԡ���� ���Ⱦ��ȣ����ڿ��� ������ƽ ��������м�������ˮ��û���壬��ˮ��Ȼ���£��ظ�����2��3�� BC 65.1%
��������
��ͼ��֪��������ƿ�з����ķ�ӦΪ�ڷ���������������£�50%�����뻷������ˮԡ���ȵ������·���������ԭ��Ӧ���ɼ����ᡢһ��������ˮ��һ��������װ���п����е�������Ӧ���ɶ�����������Ӧ���ɵ�һ�������Ͷ�����������ͨ����ƿ�б�ˮ���գ�ͨ��������ͨ�������ʹһ������ת��Ϊ������������Ӧ�������ͨ���ձ��б�����������Һ��ȫ���գ���ֹ��Ⱦ���������е��õĸ���ܺ�©�����ֹ���������á�
��1����ͼ��֪������A������Ϊ��ѹ��Һ©�����Һ©��������B����ʢװ���Լ�Ϊ����������Һ��Ŀ��������һ�������Ͷ����������ʴ�Ϊ����ѹ��Һ©�����Һ©����NaOH��Һ��
��2���������Ӧ����Ϊ�¶�Ϊ80~90������Ӧ�¶ȵ���ˮ�ķе㣬Ӧѡ��ˮԡ���ȣ�ˮԡ���ȵ��ŵ������Ⱦ��ȣ����ڿ��ƣ��ʴ�Ϊ��ˮԡ���ȣ����Ⱦ��ȣ����ڿ��ƣ�
��3��������ƽ������0.01g����泥���ʵ���г�������淋�����Ӧѡ�õ�����ƽ���ʴ�Ϊ��������ƽ��
��4����ѹ���˺�ľ�������ˮϴ�ӵIJ�������Ϊ��������м�������ˮ��û���壬��ˮ��Ȼ���£��ظ�����2��3�Σ��ʴ�Ϊ����������м�������ˮ��û���壬��ˮ��Ȼ���£��ظ�����2��3�Σ�
��5��A.������Ϊ��Ԫ���ᣬ������������Һ��ȫ��Ӧ���ɵļ���������Һ�ʼ��ԣ�Ӧѡ�÷�̪��ָʾ������ʹ�ü�����ָʾ������ʹʵ�������������Ʊ���Һ�����ƫС���ʴ���
B.���ζ�ǰ���Ӷ������ζ������Ӷ�������ʹʵ�������������Ʊ���Һ�����ƫ����ȷ��
C.ʵ���õļ�ʽ�ζ�ˮϴ���δ��ϴ����ʹ����������Һ��Ũ�ȼ�С������ʵ�������������Ʊ���Һ�����ƫ����ȷ��
BC��ȷ���ʴ�Ϊ��BC��
��6���������֪����������ʵ���Ϊ0.12mol�������������ʵ���Ϊ0.02mol���ɷ���ʽ3 ![]() ��8HNO3�ɵã���Ӧ�������������������ȫ��Ӧ��0.02mol��������ȫ��Ӧ����0.02mol�����ᣬ����IJ���Ϊ
��8HNO3�ɵã���Ӧ�������������������ȫ��Ӧ��0.02mol��������ȫ��Ӧ����0.02mol�����ᣬ����IJ���Ϊ![]() ��100%��65.1%���ʴ�Ϊ��65.1%��
��100%��65.1%���ʴ�Ϊ��65.1%��

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�