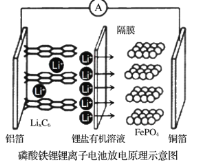
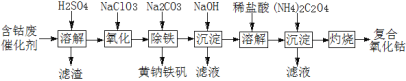
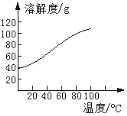
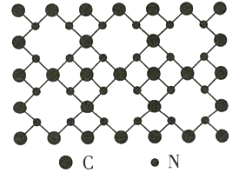
��Ŀ����
����Ŀ��ij��ȤС��ͬѧ������ȩ��������Ӧ��ʵ������������£�
A.���Թ�����ע������NaOH��Һ����Ȼ�������С���NaOH��Һ��ȥ����������ˮϴ���Թܱ��á�
B.��ϴ�����Թ�������������Һ��
C.���Թܱڼ�����ȩϡ��Һ��
D.���ȡ���ش��������⣺
(1)����A�м�NaOH��Һ��������е�Ŀ���ǣ�________________________��
(2)����DӦѡ��ļ��ȷ�����_________��������װ�ñ�ţ�
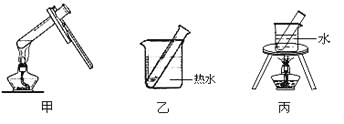
(3)��ȩ����������Ӧ�Ļ�ѧ����ʽΪ��_______________________________��
(4)����ȤС���ͬѧ������ȩ����������Ӧ�����ʵ������������̽��������ʵ���������±�����
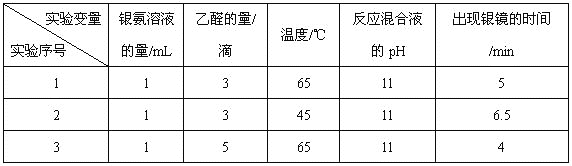
��ʵ��1��ʵ��2��̽������_________________________________��
�ڵ�������Һ����Ϊ1 mL����ȩ����Ϊ3�Σ��¶�Ϊ55�棬��Ӧ���ҺpHΪ11ʱ������������ʱ��Ϊ_________________________min�����Χ��
������Ϊ̽����ȩ����������Ӧ��������������˲ⶨ�������ֵ�ʱ���⣬����Ҫ�Ƚϲ�ͬ�������γɵ�������____________________��
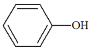
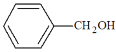
���𰸡�ȥ���Թ��ڱڵ����ۣ���֤�Թܽྻ �� CH3CHO + 2Ag(NH3)2OH![]() CH3COONH4+ 2Ag��+ 3NH3+ H2O ���¶ȶԷ�Ӧ���ʵ�Ӱ�� 5��6.5 �����̶�
CH3COONH4+ 2Ag��+ 3NH3+ H2O ���¶ȶԷ�Ӧ���ʵ�Ӱ�� 5��6.5 �����̶�
��������
(1)����������Ӧ��ʵ��ʱ��Ҫ�õ��������������Թܱ���ɾ������������������ĸ��ŵõ�������������
(2)һ�����ˮԡ���ȣ���Ҫ�û���ֱ�Ӽ��ȣ������п��ܷ�����ը��
(3)��ȩ����������Ӧ�õ�����李�����ˮ��NH3��
(4)���ݿ��Ʊ�����������ʵ�顣
(1)����������Ӧ��ʵ��ʱ��Ҫ�õ��������������Թܱ���ɾ������������������ĸ��ŵõ���������������ʼ����NaOH��Һϴ���Թܣ�����ϴȥ�Թ��ڱڵ����ۣ���֤�Թܽྻ��
(2)������Һ���ȶ����ڽ���������Ӧʱ������ֱ���û�����ȣ�Ӧ�ò���ˮԡ���ȣ���ѡ�ң�
(3)��ȩ����������Ӧ�õ�����李�����ˮ��NH3����ѧ����ʽΪCH3CHO+2Ag(NH3)2OH![]() CH3COONH4+2Ag��+3NH3+H2O��
CH3COONH4+2Ag��+3NH3+H2O��
(4)��ʵ��1��ʵ��2��������������ͬ��ֻ���¶Ȳ�ͬ�����̽�������¶ȶԷ�Ӧ���ʵ�Ӱ�죻
����������һ��ʱ���¶�Խ�ߣ���ѧ��Ӧ����Խ�죬����������ʱ��Խ�̣�������Һ����Ϊ1mL����ȩ����Ϊ3�Σ��¶�Ϊ55������Ӧ���ҺpHΪ11ʱ����Ӧ�¶Ƚ���ʵ��1��ʵ��2֮�䣬�����������ʱ��Ϊ5��6.5min֮�䣻
��������Ӧ���������������ʵ��ʱ��Ҫ�����⣬Ŀ�Ļ�Ҫ�õ���������������˻���Ҫ�Ƚϲ�ͬ�������γɵ������Ĺ����̶ȡ�

����Ŀ����������װ���л���ͨ������X���ֱ���йرպʹ���K�IJ�������Ʒ����Һ�ͳ���ʯ��ˮ��������ͬ��һ����
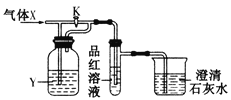
ѡ�� | A | B | C | D |
X | NO2 | SO2 | Cl2 | CO2 |
Y(����) | ŨH2SO4 | NaHCO3������Һ | Na2SO3��Һ | NaHSO3������Һ |
A.AB.BC.CD.D
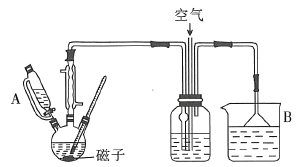
����Ŀ������ͼ��ʾװ���Ʊ����������ֲ�Ʒ(���Ⱥͼг�װ�õ�ʡ��)���й��������±���ʾ��
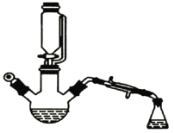
���� | C2H5OH | CH3COOH | CH3COOC2H5 |
�ܶ�g/cm3 | 0.79 | 1.05 | 0.90 |
�е�/�� | 78.3 | 118 | 77.1 |
��֪���Ҵ����Ժ��Ȼ��Ʒ�Ӧ��������ˮ��CaC12��6C2H5OH����������ƿ�ڽ��������Ҵ���������Ũ�����ϣ�Ȼ��ѹ��Һ���ߵμ��ᣬ�������õ������Ҵ��������ˮ�����������ֲ�Ʒ��
(1)����Ҵ���Ũ����ʱ��Ӧ�ȼ�����Լ���_______��Ũ�����������__________��
(2)�ߵμӴ��ᣬ���������Ŀ����_______�����ֲ�Ʒ�پ����в��辫�ƣ�
(3)Ϊ��ȥ���еĴ��ᣬ����ֲ�Ʒ�м���______(�����)��
A����ˮ�Ҵ� B��̼���Ʒ�ĩ C����ˮ������
(4)�����м��뱥���Ȼ�����Һ�������롣��Ŀ����______��
(5)Ȼ���������м�����ˮ�����ƣ������ã��Գ�ȥˮ�֡�������������������Һ�����һ�����������ƿ�ڡ���������ȥ�ͷе���֣��ռ��г�76����____��(����78������118��)֮�����ּ��ô���������������
(6)��ʵ����ԭ��������23��0mL�Ҵ���15��0mL�����ᣬ���յõ�16��0mL������������ʵ�������������IJ���Ϊ________(�ٷ�����ȷ��0.1)��